Centuries before radiometric dating was conceived and throughout much of recorded human history, scientists widely concurred that The Creation of the earth and the universe were quite recent. Scientists presumed the earth and universe to be about 6,000 years old based on calculations detailed in the book of Genesis of the bible. Additionally, the recorded genealogies going from Adam to Jesus also supported this determination. However, largely beginning in the late nineteenth century, scientists began to question the age defined by biblical accounts. Physical evidence primarily from geological formations pointed to ancient earth that did not coincide with biblical accounts’ young earth.
The concept of ancient earth was emerging due to a variety of new thoughts and observations. Paleontologists had long presumed the fossils found around the world were evidence of the Flood of Noah. But, Geologists began to believe the physical evidence appeared to have formed in the very distant past and could not be nearly young. Geologists assumed prolonged and gradual processes formed these formations over eons of time, which they eventually called uniformitarianism.
Uniformitarianism assumes that the rate of change we observe in the present represents, with but perhaps a few exceptions, the change rate throughout all history. Besides geological assumptions, other scientists were becoming naturalists. They proposed that the universe and the earth must have self-emerged and self-organized by natural laws and causes, specifically without the supernatural influence. This pivot away from the traditional biblical worldview and into a naturalistic worldview gained rapid acceptance.
In 1774, a French naturalist Georges-Louis Leclerc (Comte de Buffon), calculated the earth’s age to be about 75,000 years. By 1830, geologist Charles Lyell considered the earth to be indefinitely old. Lyell’s work greatly influenced Charles Darwin when he published his book On The Origin of Species in 1859. Darwin envisioned eons of time where mutations and natural selection worked on living organisms causing all the variety we observe today. William Thomson or Baron Kelvin in 1862 estimated the earth to be 98 million years old. During the early 1900s, Albert Einstein wanted to believe that the universe was static and infinite– that it had no beginning. However, Einstein was frustrated by the math, which continuously seemed to indicate otherwise.
Ernest Rutherford invented radiometric dating in 1905. Rutherford estimated the age of the earth to be about 2.2 billion years old from his new technology. By the 1930s, Edwin Hubble began detailing new observations from the all-new 100 inch Hooker Telescope.1 Hubble discovered a redshift of stars, which indicated an expanding universe. A universe that was not static. 1 A universe that had a beginning, later dubbed “A Big Bang.” Later, Einstein finalized the Cosmological Constant that stands today and considered his assumption of a static universe to be his greatest blunder.1 Based on many assumptions and modern technologies, the estimate of the age of the earth as of today stands at 4.55 billion years. 2
As we shall discover, any supposed mathematical precision regarding the earth’s age has been determined by many assumptions. It was not an ancient age established by radiometric dating technologies but by assumptions of naturalism. Many variances persist into modern-day radiometric dating, and one dating method renders results that often vary exponentially from one another. Many believe that radiometric dating technology serves as the end of debate regarding the earth’s age. However, as we shall illustrate, such date estimates are arbitrary and certainly unprovable. Conclusions are based on a presupposed naturalistic evolutionary history of material formations and living organisms. These assumptions form the foundation before any technology being turned on in a lab or any researcher collecting any samples.
As we discussed, scientists have estimated the earth to be as young as about 6,000 years to indefinitely old. Today it is widely accepted that the earth is 4.55 billion years old. All this variance has emerged over a relatively short period of time in the history of only about 250 years. These changes are intrinsically linked to Creation’s denial of the purposeless cause of naturalism. History had shown that scientists were convinced that the earth was ancient well before any radiometric dating technology was ever invented. It is highly suspect that the technology proposed to precisely calculate the earth’s age confirmed those same assumptions held all along.
The assumptions applied to the technology allowed the invention to measure the presupposed billions of years of age that had passed since the naturalistic formation of the earth. The invention of radiometric dating technology proved to be a self-fulfilling prophesy. The technology finally confirmed what scientists had assumed all along: the earth was billions of years old.
To keep things quite simple, think of decay as something changing over a duration of time. We generally see decay being used to describe something negative. Such as too much sugar over the years causes teeth to decay. Or, after a week, my fresh fish emits a stink of decay. Decay, as it relates to atoms, means a loss of material (an electron particle, etc.), which has to do with the ratio of protons and neutrons of a radioactive element.
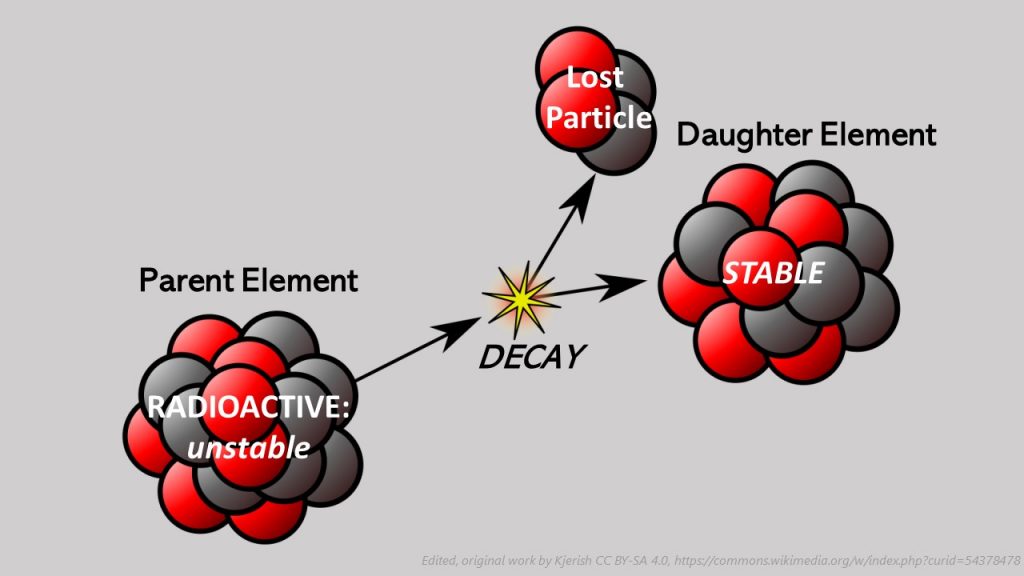
Decay means that the original element, also called the Parent, decays (loses material), causing the Parent to decay into a different element called the Daughter. Decay occurs when the Parent element that is unstable, meaning radioactive, changes or decays into the stable Daughter element. Radioactive (unstable) Parent elements decay into Daughter elements.
Radiometric dating is a technology that measures, or more accurately counts, how much of an element remains in a sample and creates a ratio of Parent to Daughter elements. The Daughter element is always presumed to have resulted as a direct decay of the Parent within the same sample. The ratio of Parent to Daughter is used to estimate the approximate age of the sample tested. The ages are based on calculations produced by assumptions, including a duration of time called a half-life.
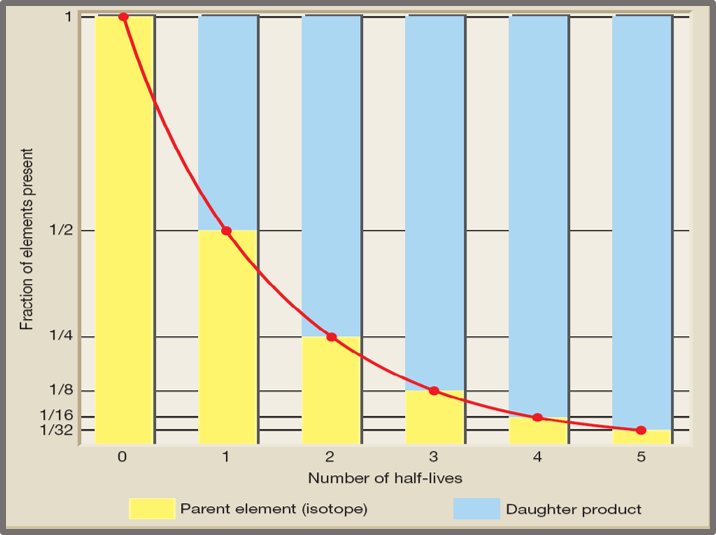
A half-life is a bit tricky to understand initially, but it is a simple concept. A half-life is the duration of time for half of the parent element to decay into the daughter element. Some half-lives are less than a second, and others are many millions of years. This means that after one half-life duration, half of the material has decayed from 100% the Parent into the daughter element: now 50% parent- 50% daughter. Here is the key: after the next half-life interval, the half that remained in the Parent element decays into the daughter: so now 25% parent – 75% daughter. After the third interval: 12.5% parent- 62.5% daughter; after the fourth interval 6.25% Parent-93.75 Daughter; after the fifth interval 3.125% Parent- 96.875% Daughter. After the 4th half-life interval, magnitudes decrease because the differences between the Parent levels are tiny.
Carbon-Nitrogen dating is more commonly known simply as carbon dating or also called radioactive Carbon-14. This dating method is critical because the unstable Parent is found in all organic life forms. Therefore, this dating method is used to estimate the age of once-living things.3 Carbon-14 dating measures the presumed rate of decay in organic samples. Radioactive (unstable Parent element) carbon-14 is formed in the upper atmosphere when cosmic sun rays collide with ordinary Carbon-13, which adds an unstable isotope forming Carbon-14. Along with common Carbon-13, Carbon-14 mixes into the atmosphere, dissolves in the oceans, and enters plants’ flesh through the photosynthesis process. Additionally, through these same mediums of the air, water, and plants, Carbon-14 enters the animals’ bodies that ate the plants (or other animals that ate the plants). Carbon-14 becomes trapped inside their flesh, and it is assumed to be continuously renewed and maintained at a steady level (emphasis on assumed). After the animal dies, no new carbon-14 can be added because no replenishment of eating happens. As the years’ pass, the unstable radioactive element Carbon-14 decays into the stable Daughter element Nitrogen.4 Carbon-14 has a concise, measurable life with only a 5,730 half-year decay rate.

Like Carbon-Nitrogen dating, radioactive elements are also measured based on radioactive Parent elements’ decay into stable Daughter elements. Some radioactive elements decay extremely fast, decaying in mere fractions of a single second. Other elements decay much slow, based on calculations, would take many millions of years to complete even one half-life.
One aspect all decay rate processes share is unpredictability. Radioactivity does not occur like the clicking of the seconds on a clock– sometimes it is a bit faster or slower. This is why the half-life measurement is used. The half-life method establishes a more reliable probability that the element’s atomic structure will have decayed as anticipated.
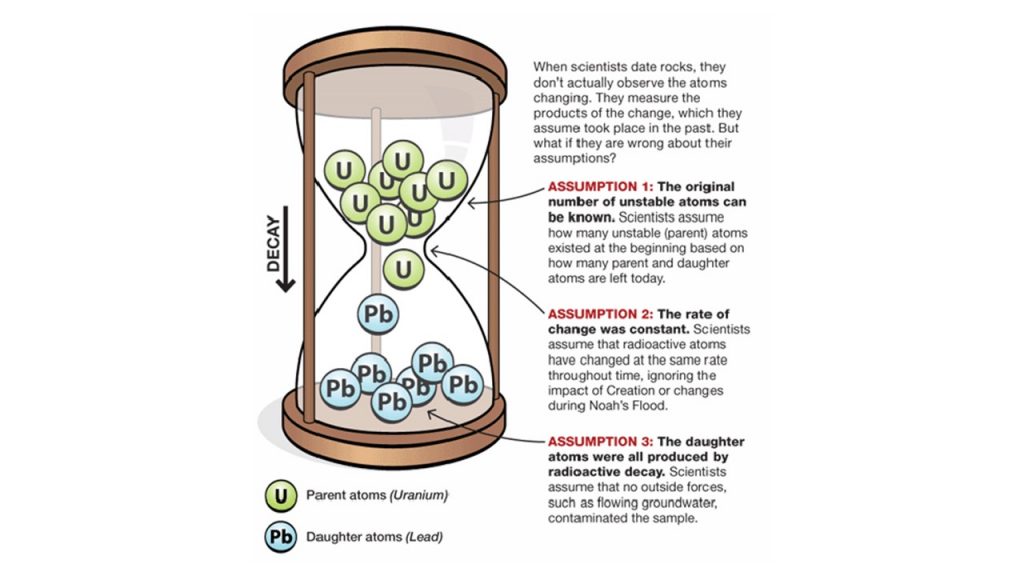
Scientifically, rocks can be measured in many ways, such as density, composition, hardness, color, texture, and more. However, contrary to the many charts that fill geological and paleontological indexes and evolutionary textbooks, age cannot be directly measured in any rock. The age of rocks can be deduced based on radiometric dating experiments, but many assumptions made by the researcher derives these calculations. Based on the researcher’s starting assumptions, effectively any age desired or anticipated can be “accurately” calculated.
“Contrary to the impression that we are given, radiometric dating does not prove that the Earth is millions of years old. The vast age has simply been assumed.”
Vardiman, L., Snelling, A.A. and Chaffin, E.F.,
When completing the form submitted with the sample to be tested, the laboratory asks the researcher to estimate the sample’s expected age before any examination. The lab then knows which results are “most” accurate to provide to the researchers. However, should samples conclude ages unacceptable or outside the exceptions age presumed by the researchers, they are discarded.4
“In conventional interpretation of…age data, it is common to discard ages which are substantially too high or too low compared with the rest of the group or with other available data such as the geological time scale.“
Dr. Hayatsu, “K-Ar Isochron Age of the North Mountain Basalt, Nova Scotia,” Canadian Journal of Earth Sciences, Vol. 16, April, 1979, p. 973-975
To illustrate, the radiometric dating method for Potassium-Argon is used to estimate the ages of lava flows. Liquid samples (before they solidify) are presumed to have zero Argon. Argon is a gas, and at scorching temperatures of liquid lava, all Argon is forced out. Therefore, fresh lava flows immediately after solidifying are presumed to be 100% Parent element of Potassium with 0% Daughter Argon. Potassium-Argon has an incredible 1.3-billion-year half-life. Therefore, if any Argon is found in the lava sample, ages amass millions of years quickly. One such example is found in rock samples were collected from a freshly solidified lava dome observed to form at the Mount St Helen’s eruption in June of 1980. The lab conducted Potassium-Argon radiometric testing that calculated the lava sample to be approximately 350,000 years old.5 Yet, the sample was only 10 years old at the time of the test. There are many such examples. Many are far worse, calculating recent lava flows as being many millions of years ancient.
“We’re building a new generation of fairy castles and myths for the next generation to play with.”
Houtermans, F.G., The Physical Principles of Geochronology, No. 151, p. 242, 1966.

To demonstrate how assumptions impact calculations, consider a hypothetical scenario of a “hot tub” spa filled with water. Our hypothetical spa holds 1,000 gallons of water in the backyard of a home filling with water. The spa is found filling, and the drain is inspected and found not to be leaking. The tub is determined to be exactly half full, containing 500 gallons of water. The research team measures the water flow coming out of the hose at a precise rate of 100 gallons per hour. Therefore, the math is quite simple. With extreme confidence, your team concludes that the spa has been filling precisely 5 hours, and in another 5 hours, it will be full. These calculations seem straightforward enough, but they are loaded with assumptions.
First, researchers assumed the starting condition of the spa. They assumed the spa tub was empty despite never observing it as such. This starting condition cannot be certain- it was presumed. Second, researchers assumed that the rate of the water flow had remained constant throughout time. The flow rate of the past, which was not observed—was also presumed. Did the rate maintain 100 gallons per hour? Was there a kink in the hose that made the flow slower in the past? Did it run faster and slow down? Third, had other influences that might have altered our spa tub calculation? Consider the drain, it was closed when it was measured, but might it have been accidentally left open during the filling earlier? What about unexpected conditions that may have affected our calculations? Perhaps, rain fell for the first hour. Perhaps the sun caused excessive evaporation that requires a mathematical adjustment.
Perhaps the hypothetical spa arguably has an exceptionally low likelihood that our assumptions would be impacting our calculations to any large extent. However, what if we apply these same assumptions toward measurements presumed to take the place of thousands, millions, or even billions of years? Obviously, any single wrong assumption would become fatal to our conclusions. Obviously, the longer the duration to be measured, the greater opportunities emerge of both the unknown events and contaminating factors that impact calculations. Nature repeatedly witnesses both uniformitarian slow and steady changes and sudden violent catastrophes. Simply put, any presumed calculation of millions or billions of years cannot be relied upon. While our measurements and technologies are maybe massively precise in the present, the unobservable past remains filled with unknowns that render calculations to be mere fantasy.
From our example, we can illuminate problems associated with any dating method. The first assumption begins with the starting conditions of our sample. Like the researchers assumed the spa was empty before filling, researchers assume there are only Parent elements with radiometric dating. The sample is empty of any Daughter element. The second assumption is the rate of flow. Researchers assume that the decay rate has remained the same throughout time, as observed in the present. Essentially, the sample has remained within a closed system free of outside influences that may have impacted the decay rate. The third assumption is that from the beginning until the present, it has continued without any contamination when measured. These assumptions, just as with our spa example, are uncertain and unprovable. They are assumptions. Clearly, no researcher observed the initial conditions or the flow rate throughout time (many millions of years). Of course, no one can confirm that there has been no subsequent contamination since the beginning.6
Despite the weaknesses of assumptions, radiometric dating promises to precisely date rocks, fossils, and even the earth. As we shall discuss in a moment, there are many weaknesses specific to different dating techniques, but assumptions singlehandedly discredit all of them. The weakest points of radiometric dating are: (a) assumptions of starting conditions of the original concentration of atomic elements are based on estimates made by the researcher in the present; (b) assumptions of rate are presupposed to be at a constant decay rate based on observations made in the present. Recent experimentation has brought isotope decay rate constants under question 7; (c) assumptions of a closed system (which do not exist in nature)3; (d) the assumption that no contamination has impacted the sample; and (e) The assumption that no unexpected or “unknown” conditions have impacted calculations. Because this assumption is based on unknown conditions, it cannot be known or accounted for by the researcher.
Such unknowns might be massive gravity during planetary formation, heat from earth molten core, asteroid impacts, solar flares, or the impact of cold temperatures. It has been demonstrated that molecular orbits cease to move when placed in absolute zero temperatures as found in space. No scientist denies that there were many events in the past that could cause such conditions. Consider Creation (The Big Bang), asteroid impacts, earthquakes, tsunamis, volcanoes, flooding, solar flares, fires, etc.
The field of radiometric dating persists despite these mountains of assumptions. Each calculation assumes universal constants that are assumed, constant decay rates that are assumed, and original conditions, including those assumed. From these assumptions, all data is calculated based on processes observed in the present—just like our spa tub example. To keep all the results tight, any calculation that falls outside the researcher’s expectations is deemed either wrong, contaminated, or unintelligible. 7
“In general, dates in the ‘correct ballpark’ are assumed to be correct and are published, but those in disagreement with other data are seldom published nor are discrepancies fully explained.”
Mauger, R.L., Contributions to Geology 15:37, 1977.
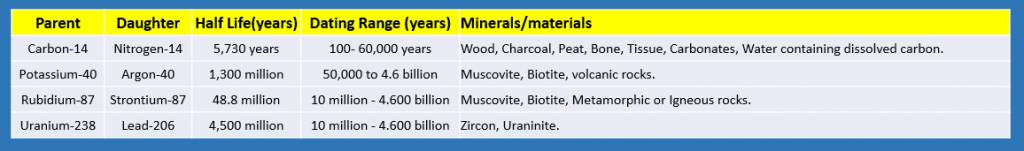
There are many different radiometric dating methods used in science to determine the age of rocks and minerals. Each radiometric Parent to Daughter has various constraints and limitations. Generally, the researcher’s dating method is based on the available rocks and minerals that can be tested.
Four primary dating methods are used to date a spectrum of materials and ages. 1) carbon-nitrogen, which is uniquely used to date Carbon life forms thought to reach about 60,000 years ago; 2) Potassium-Argon largely for lava samples 50,000 years to 4.6 billion years old; 3) Rubidium-Strontium dates rocks 10 million to 4.6 billion years old, and 4) Uranium-Lead dates granite rocks and crystals found in granite from 10 million to 4.6 billion years old.
Carbon-Nitrogen dating, or more commonly called “Carbon Dating,” estimates the age of once-living organisms such as ice age fossils, petroleum, or plants. Recall the process that forms radioactive Carbon-14 occurs when solar rays collide with ordinary Carbon-13 in the atmosphere. Carbon-14 dating methods rely on many additional assumptions beyond those discussed earlier.
One primary additional assumption is that the earth’s atmosphere has remained constant throughout the last 60,000 years. This atmospheric consistency would then result in an equal amount of Carbon-14 in the atmosphere today compared to the more distant past. However, this is known not to be the case. The earth’s atmosphere is not and has not been measured as constant, even as measured year over year. The changing atmosphere grossly distorts Carbon-14 production in the present by greatly increasing production.
One factor that impacts the atmosphere is the earth’s magnetic field, which has been measurably weakening each year for many decades. The earth’s magnetic field provides a protective atmosphere that protects the earth’s surface from harmful solar radiation. Clearly, a weakened magnetic field increases solar penetration, which increases Carbon-14 production more in the present than in the past. Yet calculations for Carbon-Nitrogen radiometric dating rely upon a Carbon-14 constant in the formula. This error causes calculations to erroneously illuminate much older dates for items from the ice age, which started with less Carbon-14 from the start causing a seeming result of antiquity.
Many other factors impact the atmosphere and solar radiation levels, including the ozone layer 8, the strengthening sun over millions of years 9, assuming that plants have not even evolved until 470 million years ago 10. Dryland to oceanic water ratios impacts global absorption rates because water absorbs Carbon-14 and dry land does not.
Shockingly, the last two decades have revealed unimaginable materials yet present in dinosaur fossils thought to be many millions of years old. Discoveries of dinosaur fossils revealed under the microscope soft tissues, including red blood cells, skin cells, and Carbon-14. These findings shocked paleontology and raised major questions about the presumed timelines of dinosaurs.
As we discussed, Carbon dating has an expected maximum range of about 60,000 years. This means after about 60,000 years, dinosaur fossils could not contain any Carbon-14 as it would have decayed long ago. Therefore, dinosaurs are assumed to have gone extinct more than 65 million years ago, but their fossils contain Carbon-14, which can be no more than 60,000 years old.
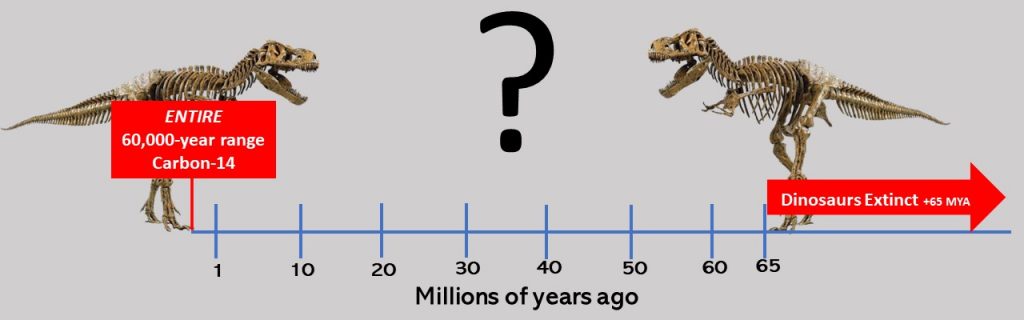
Obviously, both assumptions cannot be correct: at least 65 million years old and less than 60,000. Of course, as we discussed earlier, scientists discard any data conclusion that does not meet their presumed dates—this is what they do with dinosaur Carbon-14—they discard it as contamination. Counterintuitively, despite the presence of soft tissues in dinosaur fossils, paleontologists continue to refuse to Carbon date samples because they are assumed as being much too old.

A study tested seven dinosaur bones, including a Triceratops from Montana, hadrosaurids, a cartilaginous paddlefish, a bony fish, and fresh-looking wood and lizard bones from Permian layers in Canada and Oklahoma. Five different commercial and academic laboratories detected Carbon-14 in all the samples. The team also compared the results to several dozen published Carbon-14 results for fossils, wood, and coal from all over the world and throughout the geologic column. Comparable amounts of radiocarbon showed up in almost 50 total samples.12
Fossil wood from a quarry near Banbury, England, was dated using the carbon-14 method.6 The sample had an age calculated of approximately 20,700 to 28,800 years old. The limestone in which the wood was found was determined to be 183 million years old from the Jurassic period.
Such results clarify that it is the dating methods in conflict and are erroneous and not the presence of Carbon-14.
Rubidium-strontium dating is a method used to estimate the age of rocks, minerals, and meteorites. The Parent unstable isotope Rubidium-87 is presumed to have been present in the rock at its formation. This Parent decays into the stable isotope Strontium-87. The method is thought to be best used to date rocks, including meteorites, which are assumed to be incredibly old because the decay rate is prolonged with a remarkable 48 million-year half-life. 9
The largest unique problem with this dating method outside all the assumptions applied to all radiometric dating methods) is the reality that most minerals that contain Rubidium also have preexisting Strontium incorporated into their structure before any decay. This frustrates starting conditions and requires additional assumptions from the researcher. The researchers are left to determine when the mineral was formed and thereby make an arbitrary mathematical correction to account for this assumed initial level of strontium in the sample. Of course, this adjustment, like the presumed formation date, and all the other assumptions, are subjective.
Compound these subjective adjustments under the light of an almost unimaginable half-life of 48 billion years, and things get messy fast. To put it in perspective, the modern conjecture as an estimate for the entire universe’s age is only 13.8 billion years; therefore, the additional presence of even a tiny amount of Strontium piles on billions of years of age. Clearly, seeing some Daughter element was there from the beginning destroys the accuracy and precision because it is left reliant upon the researcher’s starting condition assumptions. So the researcher measuring the age of the sample effectively inputs the very age the method was thought to determine. Therefore, the Rubidium-strontium’s largest weakness is this required adjustments of the Daughter element (Strontium).
Additionally, North Carolina State University, in January of 2017, discovered that other “oversights” were causing samples using the Rubidium-Strontium dating were vastly overestimating ages. The reason is quite technical, but it is based on the idea that the parent element’s presumed initial portion was assumed to always decay into the stable Daughter element, which turns out not always to be the case.
The study found that the diffusion rate (rate of decay) was not constant because it depended on where the sample was found. Samples tested from cracks decayed at measurably different rates than those found in surface areas. While the differences were small– the massive half-lives made the results exponentially erroneous. The professor on the study, Robert Hayes, explained that “there’s not a simple equation that can be applied to every circumstance.” Meaning that there are no reliable assumptions that the researcher could apply that might help Rubidium-strontium be reliable. The method is hugely flawed.
“It’s a pain in the neck…If we don’t account for differential mass diffusion (decay rates), we really have no idea how accurate a radioisotope date actually is.”
Robert B. Hayes associate professor of nuclear engineering at NC State. Some Mathematical and Geophysical Considerations in Radioisotope Dating Applications. Nuclear Technology, 2017; 197 (2) DOI: 10.13182/NT16-98
The Potassium-Argon dating method is perhaps the most widely applied technique of radiometric dating used on rocks because lava flows have occurred worldwide. The method is used primarily on rocks formed by volcanic activity such as lava, micas, clay minerals, tephra, and evaporites. 11 Because Potassium is a component found in these many common minerals, it can be used to deduce the ages of other rock layers such as igneous and metamorphic rocks found between volcanic layering. The Potassium-Argon dating method is calculated by the measurement of the accumulation of Argon in a mineral.3 In these materials, the decay product Argon (a gas) escapes or is even forced out of the liquid (molten) rock material. However, when the minerals begin to solidify (recrystallize), Argon begins to form by the decay of Potassium.
Assumptions required for the accuracy of the Potassium-Argon dating technique require a closed system. No potassium contamination was gained, nor was potassium washed out. Finally, the assumed Argon in the atmosphere is deducted (did not form by decay), assumed as an all-time constant.13
First, the assumptions required for accurate calculations reveal this method’s major weaknesses because closed systems are impossible to occur in nature. Nowhere on earth can any researcher be confident that their sample remained in a closed system—especially for millions and millions of years.
Second, Argon exists in our atmosphere outside any decay processes in the sample. Therefore, Argon levels present in the atmosphere (apart from the decay process) must be adjusted from the researcher’s math formulas based on starting condition assumptions. These adjustments are again based on assumptions of levels as measured in the present.
Third, it has been well established that water can wash over potassium-rich deposits and reduce the total levels by as much as 80%. Water washing away Potassium effectively artificially increases Argon’s ratio in samples by increasing Argon ratios, which result in older date calculations. This also means that Potassium can be easily transported into surrounding minerals, causing contamination of other surrounding samples.
“Potassium (K) is lost by 80% with only 4 ½ hours of distilled water being washed over the specimen”.
http://www.cs.unc.edu/~plaisted/ce/dating.html
Other known errors came from the observed lava flows at Mount Ngauruhoe in New Zealand. The flow from February 11, 1949, was erroneously dated as 270,000 to 1 million years old; June 4, 1954, dated 270,000 to 3.5 million years old; February 19, 1975, dated 290,000 to 1 million years old. There are many such examples.
“In conventional interpretations of Potassium-Argon age data, it is common to discard ages which are substantially too high or too low compared with the rest of the group or with other available data, such as the geological time scale. The discrepancies between the rejected and the accepted are arbitrary…”
Hayalsu, A., Canad. J. Earth Sciences 16:974, 1979.
To combat this evidence and save this important dating method, evolutionary geologists have added the caveat that Potassium-Argon dating is not valid early. That is, it has been adjusted (assumptively) not to be accurate for the first 50,000 years. Other sites say not valid for the first six thousand years to cover recorded human history and ironically the approximate age of the earth based on biblical young-earth creationists.
These carve-outs are very suspect and also revealing. They are not based on measurable perimeters of science or math because the Argon is either in the sample or not. This cut out is added to resolve all that nasty data that negates all the important work of evolutionary researchers. This carves out effectively states that any recent volcanic flows as either observed or recorded within human history are too early to calculate by this method. Such flows contain Argon, and they should not, but calculations are either wrong, contaminated, or unintelligible. However, those ancient lava flows from the assumed distant past are completely reliable and accurate. To get this straight, those lava flows that are factually known give unintelligible results, but those samples collected from antiquity are accurate. How can we be asked to trust dating methods proven incorrect in the observable present to correct ancient lava formations of the distant past?
A number of processes could cause the parent substance to be depleted at the top of the magma chamber, or the daughter product to be enriched, both of which would cause the lava erupting earlier to appear very old according to radiometric dating, and lava erupting later to appear younger.
http://www.cs.unc.edu/~plaisted/ce/dating2.html
Like all the radiometric dating methods mentioned earlier, Uranium-Lead shares many of the same problems associated with assumptions controlled by the researchers. First, a unique problem to this dating method has been revealed in the discovery that Uranium decay rates are actually not constant. It turns out that decay rates vary depending on which radioactive isotope is measured from the sample– different rates can be found within the same sample!
The sample with minor differences, such as where they were extricated from the sample, amounts to massive dating differences. Despite the revelation that different isotopes have different decay rates within the same sample, the ratios are presumed to be constant by conventional geologists. 6
Second, it is widely known that Uranium, like Potassium, is highly water-soluble and can be washed out of the sample by contamination. Lead certainly is not water-soluble, so this makes the ratios appear artificially older. Compound this with the reality that the Uranium-lead has a 4.5-Billion-year half-life, which means the detection of any daughter atoms will cause the material’s calculated age to skyrocket. Massive half-lives make the measurement extremely sensitive. Each assumption made by the researcher greatly impacts the date calculations, giving the researcher latitude to calculate any age desired.
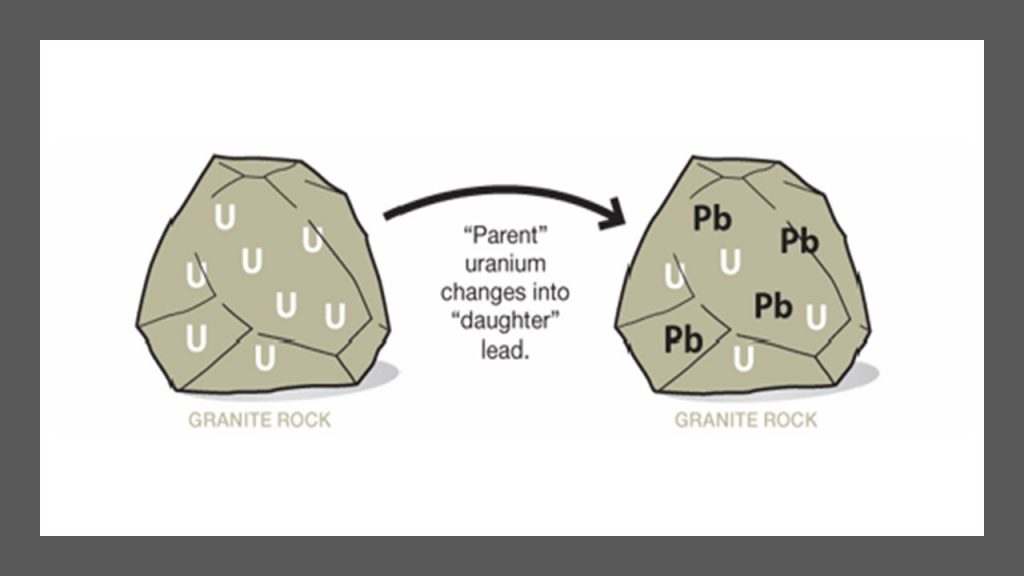
Third, The Institute for Creation Research conducted a study called Radioisotopes and the Age of The Earth (RATE), which successfully identified pitfalls in the Uranium-Lead radiometric dating method. RATE demonstrated that radiometric decay rates have not always been constant as measured at the present rates. Evidence indicates that decay rates have varied under extremes throughout time.6 When radioactive isotopes decay in rock, various gases are produced as a byproduct. As an example, the mineral zircon.

Zircon is found all over the earth’s crust and is therefore thought to be material left from the earth’s formation. These samples are assumed to be as old as the earth itself: 4.54 billion years old.
The rate also discovered that these presumed ancient crystals still contained Helium. Helium is an exceedingly small and light gas. It is rare to find Helium in any minerals or rock formations because it readily escapes. Helium is produced as a by-product of the decay process when Uranium spins off ions (decays) and becomes Lead.
The rate at which the gases escape is highly dependent on the temperature of the minerals. For example, the rate of helium diffusion at the hot temperatures 15,000 feet below the surface is about 160 times faster than the rate at the cooler temperatures at 4,000 feet. Consequently, rock deep in the crust of the earth will be more depleted in helium than rock near the surface.”
https://www.icr.org/article/both-argon-helium-diffusion-rates-indicate
Therefore, Helium’s presence shocked researchers because it was widely thought that after many billions of years, all trapped Helium would be long gone. However, after a presumed 4.54 billion years, Helium was still present.
“Surprising evolutionists, a significant amount of helium was present inside the zircons which is empirical evidence that these formations must be young. “
https://answersingenesis.org/age-of-the-earth/6-helium-in-radioactive-rocks/ the absolute quantity of 204Pb in samples cannot be measured with certainty.
Forth, RATE also found that other radioactive elements within the crystal had much shorter decay rates inside granite samples drilled from the deepest rock layers of the Precambrian, zircon crystals samples were gathered. The samples were found to have “burn marks” caused by decay (when ions escape the Parent element). These burn marks, leaving a trace called pleochroic or radiohalos. The crystal lattice structure of zircon contains several radioactive elements.

One such radioactive element is Polonium, which has a half-life of mere seconds. Polonium’s presence creates an apparent paradox; like Carbon-14 in dinosaur fossils, both Uranium and Polonium being encased together seemed impossible. Impossible under the worldview of long geologic formations forming slowly. How could the same crystal have both incredibly ancient Uranium and incredibly young Polonium in the same sample? One possible solution suggests that perhaps the liquid granite created the zircon crystal while cooling. This happened suddenly as both were created together instantaneously during Creation.
“As in the case with radiometric ages determined from almost any rock is impossible to establish unequivocally…”
Barton Jr, I.M., Canad. J. Earth Sciences 14:1641, 1977.
“Unfortunately, such checks (persistent problems) have painted a generally gloomy picture for… the (radiometric dating) tool.”
Encyclopedia Britannica; Parentheses are mine.
SOURCES
1 “Einstein’s Lost Theory Describes a Universe Without a Big Bang” By Amir Aczel Mar, 2014 https://www.discovermagazine.com/the-sciences/einsteins-lost-theory-describes-a-universe-without-a-big-bang
2 “Changing Views of the History of the Earth” by Richard Harter Copyright © 1998-2005; last Update June, 1998 http://www.talkorigins.org/faqs/geohist.html
3 University of Waikato “Geological Time” https://sci.waikato.ac.nz/evolution/RadiometricDating.shtml
4 “The way it really is: little-known facts about radiometric dating. Long-age geologists will not accept a radiometric date unless it matches their pre-existing expectations.” by Tas Walker
5 Austin, S.A., Excess argon within mineral concentrates from the new dacite lava dome at Mount St Helens Volcano, Journal of Creation 10(3):335–343, 1996; creation.com/lavadome.
6 “Uranium-Lead (U-Pb) Radioisotope Dating Method Problems First Problem: Common Lead” by Troy Lacey on January 23, 2020 https://answersingenesis.org/geology/radiometric-dating/u-pb-radioisotope-dating-method-problems/
7 “Radiometric dating and the age of the Earth” by Ralph W. Matthews, Ph.D. https://creation.com/radiometric-dating-age-of-earth
8 “Ozone Layer” by Hannah Ritchie and Max Roser, June 2018. https://ourworldindata.org/ozone-layer
9 The Faint Young Sun Paradox: A Simplified Thermodynamic Approach
F. Angulo-Brown, Marco A. Rosales, and M. A. Barranco-Jiménez, 2012 https://doi.org/10.1155/2012/478957
10 “First land plants plunged Earth into ice age” EARTH Feb, 2012 By Michael Marshall https://www.newscientist.com/article/dn21417-first-land-plants-plunged-earth-into-ice-age/#ixzz6NQKDzL6a
11 “Rubidium-strontium dating” by The Editors of Encyclopaedia Britannica 5a https://www.britannica.com/science/rubidium-strontium-dating
12 “Carbon-14 Found in Dinosaur Fossils” By Brian Thomas, PH.D. July 2015
https://www.icr.org/article/carbon-14-found-dinosaur-fossils