The following blog is a detailed autopsy of Abiogenesis.
Abiogenesis refers to the emergence of the first life on earth as emerging from non-living chemicals. The letter “a” in abiogenesis means before, bio refers to biology, and genesis refers to the beginning. Likewise, abiotic means chemicals as they existed before any biology. Origin of life researchers within abiogenesis attempt to use presumed abiotic chemicals found on the early earth to render more complex molecules. These molecules seek to explain how non-living chemical pathways might have originally formed life on earth.
Such chemical processes must consist of only the presumed simple chemical compounds available on early earth. What the early earth might have looked like is still very speculative. Still, most researchers focus on chemicals such as ammonia, methane, oxygen, carbon dioxide, hydrogen sulfide, sulfate, water, formaldehyde, carbonate, formate, cyanide, possibly hydroxide.1
Prebiotic chemistry consists of the chemicals that are speculated to have been available on earth before any biological form had emerged on earth. Before any life existed. These chemicals are naturally occurring, mainly organic, and abundant in the universe. Prebiotic processes are not goal-oriented as nature progresses as blind spontaneous (or inevitable) processes. Processes that observably occur within chemistry. Therefore, due to these inevitable blind natural processes, any prebiotic chemistry must contend with both productive and nonproductive processes and atomic structures. This means both the targeted (although nature never actually targets anything) and the nonproductive impurities must be accounted for and accumulated during any synthesis. In other words, abiogenesis must contend with massive contamination because prebiotic earth did.
Defining life may sound like a straightforward and simple enough phenomenon to explain. However, it remains critical to clarify definitions, especially within the field of abiogenesis. What makes something alive? Certainly, no one thinks of water as being alive; merely because two hydrogen atoms bond with an oxygen atom have a bonding affinity does not in any way equate to life.
Life has been discovered to exist only within cells. Within cells, hundreds of highly coordinated and multistep reactions are fueled by energy obtained from nutrients by the cell, which is converted for growth and maintenance. Living organisms have a metabolism that provides energy to allow responsiveness to their environments for reproduction (or replication). Living cells can maintain homeostasis as a self-provided internal, physical, and chemical condition. There is growth and change evidenced within their life stages.
All living organisms share several key characteristics or functions: order, sensitivity or response to the environment, reproduction, growth and development, regulation, homeostasis, and energy processing…Organisms are highly organized, coordinated structures that consist of one or more cells.”
https://courses.lumenlearning.com/suny-wmopen-biology1/chapter/the-characteristics-of-life/
Modern science has theorized that the earth is 4.56 billion years old, plus or minus about 50 million years. This determination has been calculated using various radiometric dating methods on rocks presumed to be the earth’s oldest rocks.2 Some of these rocks include meteorites presumed to be remnants from the solar system’s formation, which corresponds to when the earth is presumed to have first formed.3 Scientists speculate about what early Earth might have looked like many billions of years ago before first life emerged; such speculations are still debated.
Early speculations such as the 1953 Miller-Urey experiment (more on this later) presumed no free oxygen in the early earth atmosphere. Such conditions are called a reducing atmosphere. Just as Miller-Urey used filtered solar light to protect their experiment, they also eliminated oxygen from their apparatus.1 The chemists understood oxygen caused an acidic reaction to any organic material (called oxidation) and was, therefore, a known killer—not a good way to begin the experiment. However, a reducing atmosphere also is quite deadly to life as it causes highly noxious methane, carbon monoxide, hydrogen sulfide, and ammonia environment. Getting life out of these simple but poisonous chemicals, as we shall see, no trivial task.
Today, most scientists agree, based on lacking geological evidence of an early reducing atmosphere4, that early earth as long ago as about 500 million years ago was precise as we find it today. Nonetheless, as we stated earlier, the origin of life researchers continues to focus on the same simple chemicals known to exist in the universe as we mentioned earlier, such as ammonia, methane, oxygen, carbon dioxide, hydrogen sulfide, sulfate, water, formaldehyde, carbonate, formate, cyanide, possibly hydroxide.
Despite the collective eye-rolling of any naturalists reading this paper, the primordial soup model continues to be the most taught and widely accepted model for explaining the origin of life. This theory (speculation) is taught in a “matter of fact” manner from grammar school into university. In a nutshell, the speculation reasons that first life must have consisted of the various simple chemicals on the prebiotic earth. These simple organic compounds, hydrocarbons, and the four biomolecules (as we discussed) gathered in a primordial soup (water) of some kind. Then, energy was added (perhaps lightning, volcanic vents, etc.) to the mixture. As eons of time passed (stirring) and presto: life inevitably emerged.
“Despite bioenergetic and thermodynamic failings the 80-year-old concept of primordial soup remains central to mainstream thinking on the origin of life,” said senior author, William Martin, an evolutionary biologist from the Institute of Botany III in Düsseldorf.
“New research rejects 80-year theory of ‘primordial soup’ as the origin of life” Feb, 2010,
Wiley-Blackwell
These claims are all well beyond the reach of any origin of life reality. As discussed earlier, assuming the macro-molecules as the building blocks is problematic as even the building blocks of these building blocks have not been solved from a prebiotic process.
A study conducted by Dr. John Narcum Ph.D. found in a study conducted by a group consisting of an average age of 38 years old, 80% college educated that 41% of these people surveyed believed scientists could create life in a lab such as a frog. Of that same group, 72% believed scientists could create life like a bacterium in the lab. Both presumptions that science can create any life whatsoever are wildly incorrect. This reveals that the layman’s perceptions of actual scientific accomplishments have been misled and are completely disproportionate to reality.
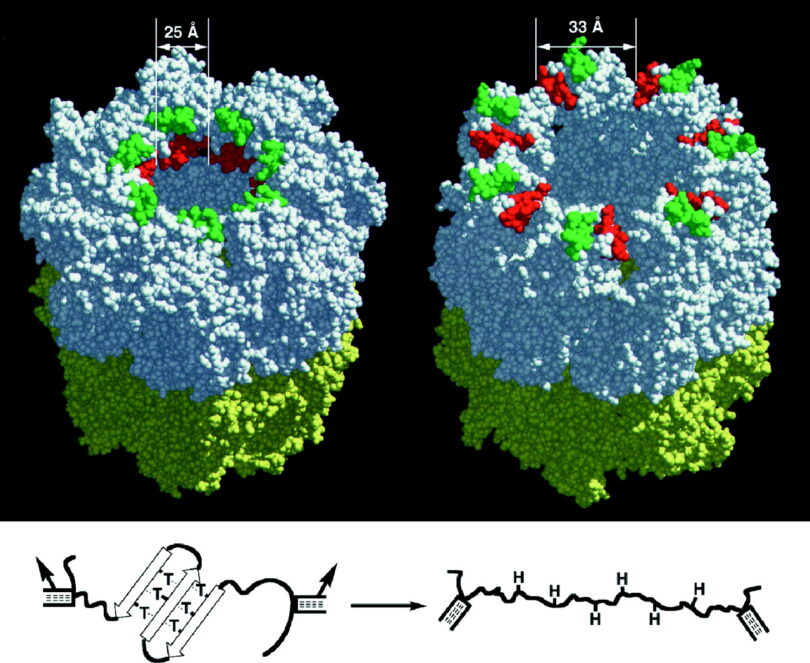
There are four classes of macromolecules that encode information within life, as observed in genetics today. These have been called the building blocks of life. These four building blocks are (1) Carbohydrates, (2) Proteins, (3) Sugars, and (4) Fatty Acids.5 Carbohydrates are also known as polysaccharides because they are comprised of monomeric sugars. Protein chains are comprised of amino acids that are folded into highly specialized three-dimensional shapes. Sugars are also known as nucleic acids, such as those found in the backbone of DNA or RNA structures. In trimeric form, such structures are called nucleotides. Fatty acids are comprised of chemical structures called lipids.
The four classes of macromolecules, as we just covered, are called the building blocks of life. However, to construct first life purely from prebiotic earth, using only simple chemicals, the origin of life researcher is tasked with building blocks of the building blocks. Scientists cannot start with these four macro-molecules because they are presumed not to exist on the prebiotic earth.
These enigmas include how did polysaccharides first form before building carbohydrates? How did amino acids first form to provide the chemical structures needed to construct the first protein chains? How did the sugars first form as used to build nucleotides as found in DNA or RNA? How did fatty acids, outside any biological process observed today form? How were the first cellular membranes formed?
Carbohydrates are macronutrients that provide energy to living cells. The energy is largely derived from photosynthesis within plants. The primary carbohydrates are comprised of carbon, hydrogen, and oxygen and become essential nutrients, including sugars, fibers, and starches.
The only known source for carbohydrates comes from life itself. Living plants convert carbon dioxide and water within the presence of sunlight into glucose (carbohydrates). Within carbohydrates, there are many structural organizations based on the arrangement of monosaccharides.
The carbohydrate is the most complex of the four macro-molecules used as the building blocks of life. A single carbohydrate (has six active sides) equates to over one trillion possible combinations.
For example, a (six-sided sugar residue” Carbohydrate) …could have more than 1 trillion possible combinations…an almost unimaginable number of possible saccharide units could be theoretically present in biological systems. Fortunately,…naturally occurring biological macromolecules contain relatively few of the possible monosaccharide units in a limited number of combinations.”
https://www.ncbi.nlm.nih.gov/books/NBK20737/?report=reader
Coupling carbohydrates used by life remain painstaking and difficult to accomplish even by the most highly trained chemists. How specialized carbohydrates could have been isolated in high yields within any proposed prebiotic system is unknown.
Carbohydrates are the hardest class of the four biomolecules to figure out how they might have spontaneously generated in a prebiotic environment. Each carbohydrate has many millions of possible chemical configurations, but only a tiny fraction of these are used by life. Carbohydrates are never even discussed by the origin of life theories because they are clueless about how this might have happened. To date, the origin of life researchers is nowhere near creating any glucose structure from a prebiotic (non-biological) method.
Building the building blocks of Proteins: Amino Acids.
All living organisms, from bacteria to human beings, are constructed of thousands of different proteins. Proteins are called the building blocks of life—but the building blocks of proteins are amino acids. Life uses twenty unique homochiral amino acids (only left-handed) in the assembly of proteins.
“…the problem (of life using only homochiral amino acids) may seem trivial at first glance, but chemists still do not understand how it happened…’no homochirality, no life‘…so far that seems to be true.
https://www.chemistryworld.com/features/the-origin-of-homochirality/9073.article
The only known source of all twenty homochiral amino acids used by all living organisms comes only from life. In other words, only biological life synthesizes its own amino acids for metabolism, or it acquires them from food sources produced by other biological organisms. Within the living cell, amino acids are chained together to construct proteins. The protein chain is ultimately folded into highly specialized three-dimensional structures. This is why mutations are so degradative–they place the incorrect amino acid into the sequence, which drives misfolding. The living cell has no idea what to do with these malformed proteins, which leads to disease, cancers, and even death.
The Miller-Urey Experiment, as discussed earlier, did successfully synthesize various racemic (mixed right- and left-handed) amino acids under the careful design and execution of the intelligently designed apparatus of highly acclaimed chemists. To be clear, this process did not produce homochiral amino acid structures suitable for life. The results were not a surprise to the chemists, which is why it was constructed as it was fashioned. Ultimately, science is no closer to offering explanations today than it was over 65 years ago.
Naturalists often criticize those who use the Miller-Urey Experiment to illustrate the weakness of a mode for abiogenesis regarding amino acid formation. They claim the experiment is merely a “creationist talking point.” They additionally point out that The Miller-Urey Experiment is a vastly outdated experiment, which is true. However, if pressed for alternative explanations to account for prebiotic amino acid formation, just as with the Primordial Soup narrative, dialog resorts back to Miller-Urey. This is proven within websites on the internet, museum displays, textbooks, and even from the lips of university professors. Clearly, there remains no better answer to the formation of amino acids than The Miller-Urey Experiment from 1953.
A polysaccharide is a complex carbohydrate polymer formed from the linkage of many monosaccharide monomers. Examples of polysaccharides are cellulose, glycogen, chitin, and starch. The best-known polysaccharides are starch which is the main form of energy in plants. Polysaccharides are used in living cells to send messages and provide support to cells and tissues.
Like amino acids require homochirality to assemble protein chains. Similarly, sugars require consistent ring structures to polymerize. They must build identical structures for DNA or RNA to build nucleotides or ribonucleic acids (identical alpha and beta chain connections). The purity for this production can only be synthesized within the living cell.
The only known source for polysaccharides is biological sources. Any attempt to derive polysaccharides from a prebiotic method has fallen short. Concepts that sugars can emerge from non-biological sources exist only in imagination. Origin of life researchers is clueless as to how sugars emerged prebiotically.
Fatty acids consist of lipids from living sources such as fruits, vegetable oils, seeds, nuts, animal fats, and fish oils. Fatty acids are not found in a free state in nature outside biological sources. While fatty acids are used in various products outside food products (biological forms) such as soaps, detergents, and cosmetics, they do not form outside living organisms. Just like carbohydrates, amino acids, and polysaccharides, lipids’ only known source is biological. Assumptions that lipids formed in a prebiotic synthesis are highly imaginative and vastly unproven.
How would a fatty acid form from methane in a prebiotic environment? Answer: completely unknown.
Chemistry contends with a massive array of compounds and atomically complex structures. Some of these compounds have mirror-like corresponding chemical compounds. As an analogy, like a left-handed and right-handed glove, they are similar but non-superimposable. Each corresponding structure has the same atomic connectivity and shape and three-dimensional mirror image shape. Such chemical compounds are called enantiomers. Chemicals that do not have such non-superimposable three-dimensional structures are called diastereomers. Furthermore, molecules with the same molecular formula and sequence of bonded atoms but differ in three-dimensional orientations are called stereoisomers.6
Together, chemical molecules persist as enantiomers, diastereomers, or stereoisomers. Each structural type provides unique and highly specialized characteristics, including connectivity, affinities, and various reactions within chemistry. One type is not inferior or superior to the other. However, most attention is paid to enantiomers within the origin of life research because these chemical structures are non-superimposable. This non-superimposable design translates into high yields with chemical purity driven within similar chirality.6
Enantiomers with mirror image structures are also called chiral molecules. These molecules often share a similar or identical stereogenic center (at least one carbon atom) but have the corresponding right- or left-handed structures. Any mixture of chiral molecules that contain both right- and left-handed structures are called racemic.7 Any chemical mixture that contains only right- or left-handed structures is called homochirality.
In all living organisms, only the left-handed homochiral (same non-superimposable three-dimensional structure) is used. Living organisms do not use any right-handed structures. Life uses twenty different left-handed homochiral amino acids to build proteins within the living cell.
To say that homochiral was not initially required is empirically false. Homochiral was required before the first life might have emerged. How biological systems might have emerged with pure homochiral amino acid structures remains an enigma. Newer research has determined that homochiral structures also provide amazing design properties to translate information within the living cell nearly at the speed of light in a process called Chiral-Induced Spin Selectivity (CISS).
Chiral-induced spin selectivity (CISS) relates to how electrons pass-thru chiral molecules. Spin up or spin down, depending on orientation. Once the state of spin is preferred (left-handed) reveals a major role for chiral structure. Matched spin electrons go through unmatched do not base on the helical pitch. Which allows the transmission of information by electrostatic means at near the speed of light.8
Quantum mechanics demonstrates that for two electrons sharing a region of space, their electrostatic repulsion energy is contingent upon whether their spins are parallel or anti-parallel. “Both electrons can be spin-up, spin-down, or one can be spin-up and the other spin-down. The CISS effect exploits the spin properties of electrons. Homochirality provides highly specialized electron transmission through the molecule based on the electron’s specialized spin.”8.
“Chemists now know that chiral molecules act as electron spin filters, permitting the one-way passage of electrons of one spin in preference to electrons of the other spin. Selective transmission probabilities can be a hundred times larger in a chiral molecule than in a non-chiral molecule. For an electron of the proper spin, chiral molecules show far less backscattering of the electron; this greatly reduces the heat released from the molecule during the electron’s passage. Lower heat affords any biological system an advantage.”8
“It was established that the efficiency of electron transport through chiral molecules depends on the electron spin and that it changes with the enantiomeric form of a molecule and the direction of the electron’s linear momentum. This property means that, for chiral molecules, the electron spin is strongly coupled to the molecular frame.”
https://pubs.acs.org/doi/10.1021/acs.accounts.0c00485
CISS answers the mystery scientists have wondered for centuries about why and how cellular organisms do not overheat. This is now known to be due to the ultra-efficient spin (“up to 99.99%” James Tour, Synthetic Chemist, from Video series “Abiogenesis”) within biochemical functions provided by CISS and its unparalleled molecular efficiency. “CISS reveals that more is going on, and it is not subtle in its influence.” 8. Unfortunately, homochirality and its use of CISS prove as an essential aspect of life. Therefore, any origin of life theory must answer how homochirality emerged within impure chemical structures.
We observe living organisms of the same species reproducing. We know that gene variants (DNA alleles) provided by the parent’s sex cells at reproduction provide such diversity to their offspring. We can also conclude that the concept of “survival of the fittest” is an excellent descriptor for life within nature. We find that the offspring that ultimately is the fittest will also be the most likely to reproduce and pass their traits to future generations. For our purposes here, we shall call this germline process Natural Selection.
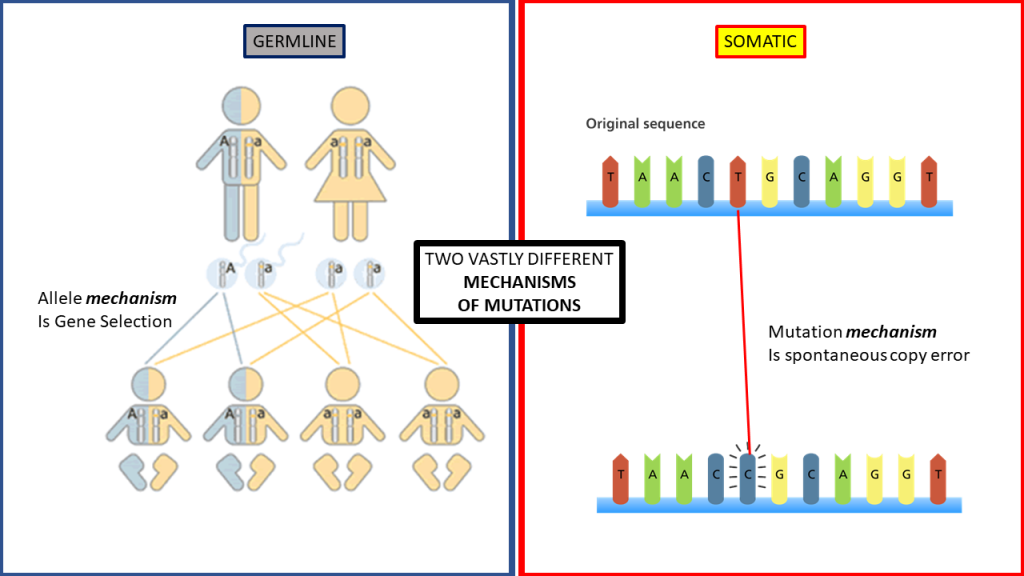
While Natural Selection can be observed within living organisms inside biology, no such process exists for molecules or chemicals because they do not have any genes. Even somatic mutations can generate changes (albeit degradative ones) chemical structures that are void of any such genetic information and, therefore, also devoid of the mechanisms of Natural Selection.
Additionally, from all the data gathered within the origin of life studies, molecules and chemicals have never been shown to tend to life or order. The concept of Natural Selection does not apply to abiotic chemistry and therefore cannot be used to justify chemical changes within abiogenesis theory.
Fatty lipids have polar groups pointing in and other non-polar ends pointing out—one outside the cell and one inside. The phospholipid bilayer is generally impermeable to ions—some uncharged molecules can readily diffuse. Substrates insoluble to hydrocarbons do not pass through the cell membranes. Cell membranes have highly specialized protein portals that allow certain chemicals in and certain chemicals out. It has never been shown that a homogeneous lipid bilayer system can operate as a suitable cell membrane—missing all the specialized functions provided by protein portals.
Living cells use thousands of different lipid structures which swarm in and out of the membrane during cellular function. In fact, lipid bilayers even surround subcellular organelles such as mitochondria which themselves are self-contained microsystem assemblies. Additionally, each lipid membrane has unique and specialized lipid composition.
Lipid bilayers have non-symmetric distribution between inner and outer surfaces. Without the protein-lipid portals, transport sites and active pumps provide the passage of ions and molecules in and out of the cell with high specificity. Simple fatty acids idealized today as potential prebiotic membranes for protocells cannot be derived. Outside biological systems, simple chemicals provided on a prebiotic Earth would fail without corresponding protein portals.
An interactome is a set of molecular interactions within a particular cell. One aspect of an interactome is the protein-to-protein combination, but this is just the tip of the iceberg. This term refers to countless physical interactions between molecules such as proteins, genes, nucleic acids, lipids, enzymes, and carbohydrates within the living cell. Together interactome is the effective operating system of the global function of the cell between all the information-carrying macro-molecules of the cell. Even the smallest organisms, such as a single cell yeast, are estimated to have perhaps 30,000 interactions. The possible variables within these 30,000 interactions within a yeast cell have been estimated to be 1079,000,000,000. This was not a misprint! Ten with seventy-nine billion zeros after it. To put this in perspective, there are an estimated 1090 elemental sub-atomic molecules in the universe. This number is massive is an understatement—it is a super, super, super, super-astronomical number.
Origin of life theorists do not discuss the origin of information because they have literally no idea. It goes without saying that this is clearly the largest hurdle for any theory of abiogenesis to overcome. Although this is glossed over time and time again, it remains the greatest mystery in biology. How it might have emerged abiotically is vastly implausible. Yet, without answering the origin of information, abiogenesis cannot proceed.
Information, such as that provided by a written digital language, exists, it is always primary, and the matter is secondary. Naturalism often concludes that only matter exists despite the massive evidence of informational bearing biological forms. As we have seen, the living cell has many informational bearing attributes, including DNA, RNA, and the many attributes of Interactome, as discussed above. As an analogy to the computer programmer, the software they write is primary and provides a purpose. The hardware is secondary.
It is not difficult to understand that random letters or repetitive sequences do not generate information. They can produce a form of order found in a snowflake or a crystal, but no specified information is provided, such as found in a digital language. We might clarify that information is translated by the highly specified order of the digital characters used to convey purpose or meaning. Language, such as that used within biological systems like DNA, can be recognized in symbols. Components of language include: Uniquely defined, irregular sequence, irregular symbol, not merely repeating sequences, some repeating of sequences, and has a clear and distinguishable structure.
The word information comes from the verb “to inform” or, more specifically, “to give form to the mind.” A careful review of information science reveals five required aspects within the origin of information, including 1) All information exists and requires a form of communication. DNA uses code as amino acid chemical symbol letters. (2) Code exists only because of deliberate action. Specified information cannot and does not emerge as inevitable or spontaneous natural processes. (3) All information requires a sender or designer, which can only be caused by the designer’s mind pragmatically deciding to do so. (4) All information derives from a mind or intelligence. There is no specified information without a mind. (5) All information is evidence of volition (the will or desire of the designer) associated with its creation.
In chemistry, not all reactions are reversible. Many reactions and interactions are largely (outside massive synthetic interventions) one way only. Other chemical reactions do not self-stop or self-isolate, especially in the presence of contamination and impurities. Much of the bonding of chemistry can be analogized to magnets with either positive charges or negative charges. Some chemicals have neutral attributes as well. Chemical linkages that are neither desirable nor conducive toward life would certainly overwhelm the experiment. This is precisely what prebiotic earth must overcome with only rocks and simple molecules—no lab.
While common descent evolution can rely on Natural Selection to use eons of time as its hero—prebiotic chemistry cannot. Why? Because most chemical reactions will randomize, as with our bucket of magnets analogy above. Time does not help this at all. In fact, time can also be the enemy concerning purity, yields, structures. Many amino acids have truly short half-lives (in the span of minutes to hours) when outside the protection of the cellular membrane. Also, the order of events is paramount in chemistry- called the “reagent” order. To help explain reagent order, consider that you can notice the cake you are making before it is first baked. Clearly, many physical parameters must transpire in exact order, such as temperature fluctuations and requirements—we cannot bake our cake in subfreezing temperatures. We also must consider pressure, light (or no light), atmospheric gases (or no gases or specifically excluded gases). We might have careful controls within a laboratory, but certainly not on the prebiotic earth.
Also, the desired purity of a certain chemical might be well known by the chemist, but not by a blind natural process under a rock somewhere on early earth. A mass transfer problem emerges when obtaining the high purity and yields required to proceed in a prebiotic chemical experiment. How can the prebiotic earth control chirality, purity, yields, reagent orders, etc.? In chemistry, impurities persist without clear and purposeful designs to eliminate or isolate undesirable interactions.
As we stated at the beginning of our paper, because a purely natural and blind process must cause abiogenesis, the origin of life researchers must contend with both the productive and non-productive (impure byproducts) generated by any supposed prebiotic process. To attempt to imagine how even amino acids could purify into using only homochiral structures defies imagination, let alone all the other information-bearing attributes of the cell.
The synthetic prowess and remarkable ingenuity of researchers and scientists are astounding. Reading the specific protocols used during their experiments reveals massively fluctuating temperatures from hot to warm to ice-cold, solvent washes, reagents, acetylene gas torches. These processes are completed in precise orders repeatedly. Fluids preserve structures in precise pH-balanced fluids, dry traps, specialized ion exchange resins, careful and extraordinarily difficult separation of ions from reaction mixtures. All these precise protocols must occur without carefully prepared products and procedures; the desired biomolecule would be destroyed.
The prebiotic earth needed to form the first living cell under a rock or a warm water body. It had no mass-transfer controls for purity and yields. It had to contend with mass racemic and stereochemistry randomly jumbled by happenstance and natural affinities. The early earth had no means to purify solutions, no vacuum pumps, no PH balancing solutions, no temperature controls, no holding devices, no glass beakers, no blowtorches, the list goes on and on. The enigma of the origin of life must ultimately contend with impure mixtures and random processes. Ideally, such experimentation should not involve a chemist in any way.
Many origins of life scientists begin with the so-called building blocks of life as macro-molecules. Even with the presumption that the prebiotic earth contained these macro-molecules outside biology, any meaningful stride toward abiogenesis is largely void. Also, as we discussed earlier, science cannot even create the building blocks needed to construct these building blocks—let alone from using only prebiotic materials. For example, before the researcher can propose how DNA or RNA emerged, they must first solve sugars and carbohydrates. Then they must explain how sugars might spontaneously emerge in pure repetitive and improbable structures by chance. One wrong sugar and the entire sequence is destroyed (must polymerize on the alpha-beta 3’ – 5’). This will not occur by happenstance and has never been observed as occurring in nature outside biology. Ribose must polymerize for structure, and then amino acids must be ordered to form proteins without informational code beyond random chance. Whoever trivializes the synthesis of nucleic acids is uniformed. Nucleotides used in experiments are derived from life and are purchased by the researcher.
Unknown to most non-chemists and those outside the origin of life, the researcher purchases the organic chemicals and macro-molecules used in abiogenesis experiments. Generally, all of these purchased materials come from biological sources. Also, the macro-molecules used are not prebiotically manufactured but come from biological sources. Therefore, the origin of life experiments is being conducted with chemical compounds which ironically are derived from the very life forms they are being purported to produce.
Both DNA and RNA are very fragile sugar macro-molecules. These structures are composed of oxygen atoms which can readily bind as to allow chaining to occur easily. This binding capacity also means that they can just as readily chain together incorrectly. For either DNA or RNA sugar backbones to be useful, they must bind together precisely. One sugar molecule incorrectly positioned destroys the macro-molecules information-bearing capacity.
Of the two molecules, DNA is more stable than RNA because it contains one less oxygen atom (deoxyribose) than RNA does (ribose). This means that RNA is more reactive and is much more likely to bond incorrectly, becoming an impurity. Again, the sugar backbones of both DNA and RNA are only useful to the information-bearing macro-molecule if precisely arranged to convey meaning. Such precision has not been observed to occur abiotically or outside the precise controls within the living cell anywhere in the universe.
Chemists that conduct experiments with RNA are instructed to store them at frigid temperatures between -20o C and -80o Celsius to prevent rapid decomposition. Additionally, these are stored in purified water-free solutions, dehydrated, or under liquid nitrogen. These careful procedures can help prevent degradation for up to one year. The product providers point out that even under these careful protocol’s RNA will still retain some reactivity. Should users want to use RNA under room temperatures, the storage is recommended to be dehydrated and held in clean stainless steel mini capsules within an anhydrous (no water) and anoxic (no oxygen) environment. Storage warnings also point out that atmospheric humidity is an additional deleterious factor and should be maintained within the laboratory. Their vendors warn the researchers that RNA is extremely sensitive. One primary sensitivity RNA has oxidation due to oxygen either in air or water (and the reactive properties of the macro-molecule). It goes without adding more to the susceptible protocols associated with the handling of RNA that any concept of this marvelous macro-molecule emerging on prebiotic earth under a rock or in a warm primordial soup falls somewhere between massively fanciful and the super-astronomically miraculous.
The researcher’s hype at least partly drives the primary force behind the misconception of abiogenesis. Other media stands ready to expand the hype into clickbait on the internet. Despite the reality, the origin of life, researchers remain clueless on building such structures prebiotically. The internet is full of articles promising otherwise. Articles from how scientists have created synthetic life in the lab to precise recipes on how life first began to burn up the internet daily.
In 2018, Nobel Laureate Jack Szostak published in the most prestigious source Nature an article “How Did Life Begin?”. Szostak explains that the early earth atmosphere likely had no free oxygen. As we discussed at the beginning of this paper, this accretion has been largely debunked due to lacking geologic evidence of a reducing atmosphere. Still, we can overlook this issue because it remains speculation. Clearly, chemists and biologists recognize that free oxygen is a genuine destroyer of organic macromolecules, and therefore oxygen is not desirable under any prebiotic model.
Szostak explains that simple elements such as oxygen (not free oxygen), nitrogen, carbon dioxide, hydrogen, water, and methane were transformed over the eons by lightening, asteroid impacts, and ultraviolet light into hydrogen cyanide (built from hydrogen, carbon, and nitrogen). The newly formed molecules of hydrogen cyanide were stirred by circulating waters washing through the prebiotic rocks of the early earth. These waters concentrated iron from the rocks into a “stew,” which eventually resulted in the molecule iron-cyanide. To this point, putting aside the reducing atmosphere debate, the chemistry remains at least plausible, but the story shifts very quickly from here into massive leaps of faith.
Szostak points out, “Life as we know it requires RNA. Some scientists believe that RNA emerged directly from these reactive chemicals, nudged along by dynamic forces or the environment.” Wow! As the comment “nudged along by dynamic forces in the environment” goes—chemists do not get to use storytelling devices. What are the chemical pathways “nudging”? Nudging does not exist in chemistry terminology. Dr. James Tour points out that the claim is “utterly ridiculous.”
It gets better. Szostak states that “eventually nucleotides formed” as the building blocks of RNA. Wow! How? Where did the macro building blocks for ribose come from? Iron-cyanide or hydrogen cyanide? How did it react to specific single directional chain binding? Where did the specified genetic information come from? Szostak makes the understatement of the century here and points out that “some stages in this process are still not well understood.” Some of the processes? How about none of the processes are understood at all! Prebiotically formed sugars have never been demonstrated.
Then, after RNA was “made,” some strands became enclosed within tiny fatty cell vesicles (protocell membranes), forming spontaneously. As we discovered with all information bearing macro-molecules, including fatty acids (lipids), they only derive from living biology. Also, the term spontaneously implies a chemical pathway from methane—this is not provided because it is completely unknown.
Finally, “As the membranes incorporated more fatty acids, they grew and divided; at the same time, internal chemical reactions drove replication of the encapsulated RNA.” Wow-wow-wow! This employs fanciful storytelling but how such chemical reactions might have occurred remains completely unknown. However, based on a casual reading of this article, one would be completely misled. This is why the population is completely deceived as to the current status of explanations of abiogenesis.
Other vastly misleading articles include “Biologists create the most lifelike artificial cells yet” from Science by Mitch Leslie Nov 2018. Which essentially involved scientists copying all of the nano-machinery of a living cell into a plastic and clay microcapsule. That is it—it is an actual living cell transplanted into an artificial microcapsule. Not close to creating anything like artificial life, such as the title of the article claims.
As a final example of deceptive hype, the Harvard Gazette published the article “Mimicking life in a chemical soup,” claiming (based as clickbait from other research papers) that “A Harvard researcher seeking a model for the earliest cells has created a system that self-assembles from a chemical soup into cell-like structures that grow, moves in response to light, replicate, and exhibit signs of rudimentary evolutionary selection.” However, under scrutiny, Dr. James Tour points out that the reaction is not seen in nature and is purely synthetic. While the synthetic polymerization was interesting and a job well done by the researchers, it provides no new pathway to the origin of life from organic chemicals.
Origin of life research depends upon many tools which can be considered cheating. As we discussed earlier, the macro-molecules used in experiments are purchased and not derived by the researcher. The Source of these macromolecules is derived from biological sources—the only known source of such structures.
In working to determine possible pathways for simple chemicals to become more synthesized, a process called relay synthesis is used. Here, the chemicals are rendered under some specific method of temperature, solvent, or energy source. The chemist has a specified outcome desired. Without highly specialized controls, the chemical is detected within impurities and contamination as shown below in scenario A. Scenario A has many impurities and only a small yield of the desired material sought by the chemist. As the chemical is purified, yields decrease. Scenario B has much of the impurity removed. Finally, scenario C at the top is the desired material needed to move to the next step in the origin of the life model.
What origin of life researchers effectively do is merely find the indication that the desired material is present and then buy that highly purified material to move to the next step. This shortcut is called relay synthesis, and it is cheating.
As we stated at the beginning of this paper, because abiogenesis was a blind natural process of nature without target or purpose before any biological form ever existed, the researcher must use both the productive and non-productive materials of chemical reactions. Ideally, the experiment would happen without any chemist involvement at all. However, using relay synthesis effectively eliminates the massive problem of contamination from non-productive materials. Additionally, relay synthesis eliminates problems with low yields as prebiotic methods usually generate. Many times, the targeted material is only in the single digits to less than 1%. Clearly, the prebiotic earth had no method to purify materials or store them in containers for use in precise reagent order.
Levinthal first proposed the protein folding paradox in the 1960s based on the enormous complexity and vast unlikelihood that proteins might randomly fold properly. Since this time, the paradox has grown exponentially under the illumination of the thousands of protein structures within the living cell. Genetic mutations cause protein misfolding which leads to dysfunction and degradative effects.
The paradox expanded into what has been called the Levinthal paradox 2.0. This paradox highlights the bewildering combinational complexity of the living cells’ molecular components from a spontaneous perspective. Researchers have determined that even the simplest living organisms, such as the single-cell yeast, have combinational complexities that are mathematically insurmountable from the non-covalent bond possible interactions. Non-covalent bonds are driven by specified information and not covalent affinities. The sum of the possible interventions within a living cell, as we discussed earlier, has been called the interactome.
As we discussed earlier, just the protein folding combinations are well outside the mathematical likelihood of the age of the earth to have ever occurred. To make matters much worse, modern genetics has determined that the variables within even the simplest living cell, such as yeast, have a combined interactome possible combinations of 1079,000,000,000 . These realities render the likelihood of any random emergence of life from prebiotic chemicals as, in the very least, irrationally fanciful.
As we have stated throughout this paper, ideally, any origin of life experiment could be conducted without any chemist whatsoever, relying only upon chemical affinities. The processes should be so fundamental that they would happen under a primordial rock under the idealized primordial soup. However, the field of abiogenesis is overwhelmed by the input of knowledgeable chemists. Chemists that operate within goal-oriented targets despite the reality that random nature is supposed to have operated blindly.
Chemists rely on help from a form of natural selection within mere chemicals despite the reality that natural selection cannot occur without biology and reproduction. Chemicals do not have any propensity to evolve toward order, let alone life. Chemicals know absolutely nothing about what is pure as opposed to what is contamination. Without a target, there is no difference. Natural chemicals are racemic and lack the required homochiral structures needed for information-bearing macro-molecules used by life.
Chemists provide massive input. They employ relay synthesis to isolate tiny traces of the material they seek. Then they purchase the purified material to move to the next step. Actual yields are quite minuscule, but this is unimportant because the relay synthesis eliminates this problem. Additionally, any need for genetically specified information such as DNA or the living cell is replaced by intelligence and, just in time, ordering sequences of the chemist.
They provide the reagent order, temperatures as needed hot or cold, pressures, light or no light, atmospheric gases or absence of gases, non-water purifying solvents, dehydration as needed, sterilized storage decanters, metallic microtubule storage devices, ice-cold washes, acetylene gas torches, PH adjustments, ion exchanges and separations, vacuum pumps, atomic reaction protections (to avoid the billions of undesirable chemical combinations), glass beakers, and simplified methods to transfer one purified chemical into another vessel. Building each experiment like a divinely precise recipe—step by step, expertly devised and executed.
Clearly and undeniably, the input of the chemist is overwhelming. In the origin of research experimentation, we must acknowledge that the prebiotic earth had none of these tools, no laboratory, no sterilized storage canisters, and not even one chemist.
“The transformation of an ensemble of appropriately chosen biological monomers (amino acids, nucleotides, etc.) into a primitive living cell capable of further evolution appears to require overcoming an information hurdle of super-astronomical proportions, an event that could not have happened within the time frame of the earth, except as we believe as a miracle.”
Hoyle and Wickramasinghe 2000
“All laboratory experiments attempting to simulate such an event have so far led to dismal failure.”
Dreamer, 2011; Walker and Wickramasinghe, 2015
To qualify as prebiotic, the components of elementary organic and inorganic compounds can be used such as ammonia, methane, oxygen, carbon dioxide, hydrogen sulfide, sulfate, water, formaldehyde, carbonate, formate, cyanide, possibly hydroxide: no homochiral amino acids, no nucleotides, no sugars, no phospholipids. Of course, no pristine lab conditions, human-designed coupling agents or protecting groups, glass vessels, solvents, degassing, vacuum pumps, no simple method to transfer one chemical to another vessel.
Popular culture, in its tireless defense of Naturalism, has effectively hijacked science. What constitutes truth or facts has become more and more about perpetuating preexisting beliefs. The concern of preserving and reporting only empirical evidence is of secondary importance. This is why well-intended researchers feed the hype by writing articles such as creating life from scratch in the lab. They report this despite this is nothing close to the truth. In reality, outside the meticulous planning and execution of massively skilled chemists, natural processes yield only a mess of randomly formed chemical structures vastly unusable for life.
Shockingly, most all origin of life chemists and researchers ignore the vital importance their very participation played in the direction, outcome, and yield of the experiment they performed. Without the input of vast intelligence and procedural protocols provided by the chemist within the world’s most sophisticated labs, we find prebiotic chemicals in nature forming nothing more than a tangled mess of chemical compounds vastly unsuitable for life.
1-“A Production of Amino Acids under Possible Primitive Earth Conditions” Stanley L. Miller May, 1953 https://abenteuer-universum.de/pdf/miller_1953.pdf
2-“How is Earth’s Age Calculated?” Jeanna Bryner LiveScience https://www.livescience.com/32321-how-is-earths-age-calculated.html
3-“Age of the Earth” USGS https://pubs.usgs.gov/gip/geotime/age.html
4-“Origins of Organic Molecules in a Non-Reducing Atmosphere” Biology LibreTexts 20.4 Jan, 2021
5-BC Campus- Concepts of Biology section 2.3; https://opentextbc.ca/biology/chapter/2-3-biological-molecules/
6-“Enantiomers vs Diastereomers vs The Same?” by James Ashenhurst; Master of Organic Chemistry https://www.masterorganicchemistry.com/2019/03/08/enantiomers-diastereomers-or-the-same-1-using-models/
7-https://www.masterorganicchemistry.com/2012/05/23/whats-a-racemic-mixture/
8-CISS https://inference-review.com/article/chiral-induced-spin-selectivity
9-