The HIV Immunity mutation
The (NON-MUTATED) CCR5 gene mediates white blood cells called leukocytes. This gene has many functions and purposes within the cell. CCR5 provides (1) protection against certain pathogens; (2) an immunopathology triggered by exacerbated inflammation; (3) ensures recruitment of leukocytes (white blood cells); and (4) the activation of antiviral pathways in the epithelial cells; (5) is crucial for the development of antiviral response and (6) the proper induction of immunologic memory; (7) contributes to a suitable immune response to deal with viral infections; (8) {while}...subsidizing excessive inflammation and tissue damage.8
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4275532/
THE GOOD: Thought to be a mutation from as long ago as 2,900 years ago (ancient assumption- see the index at the bottom). This allele variant is found in 1 in 10 people today. It has been found that it stops the spread of HIV in carriers. The CCR5 gene consists of 352 amino acids. In this CCR5 deletion, one single amino acid is mutated at the 32nd position of the gene (delta-32).1 This mutation inhibits normal cellular function, inhibiting the HIV virus and potentially other proteins from entering certain cellular surfaces. “…the presence of the mutant delta32 protein in the endoplasmic reticulum inhibits transport of the wild-type CCR5 protein to the cell surface via a trans-dominant mechanism. Because most strains of HIV use CCR5 to enter host cells, if both copies of the CCR5 gene are deleted (not just one copy), it (counterintuitively) protects against HIV infection….Individuals naturally homozygous for the delta32 mutation, which abolishes CCR5 expression, are generally healthy and at no apparent disadvantage.”2 The CCR5-delta 32 mutations hampers HIV’s ability to infiltrate immune cells (due to deleted genetic material).6
“1% of people descended from Northern Europeans, particularly Swedes, are immune to HIV infection. These lucky people are homozygous carriers of the mutated gene – meaning that they inherited a copy from both of their parents. Another 10 -15% (the number has even suggested to be 18%) of people with European heritage inherited one copy of the gene. Just one copy of the mutation does not prevent against infection. It does however reduce carrier’s chances of infection and delays the progress of AIDS. Since the CCR5-delta 32 is tied primarily to the Eurasia region, the mutation has not been found in Africans, East Asians, or Amerindians.” 6
Paoli, Julia, “HIV Resistant Mutation,” Oct, 2013, Scitable by Nature Education
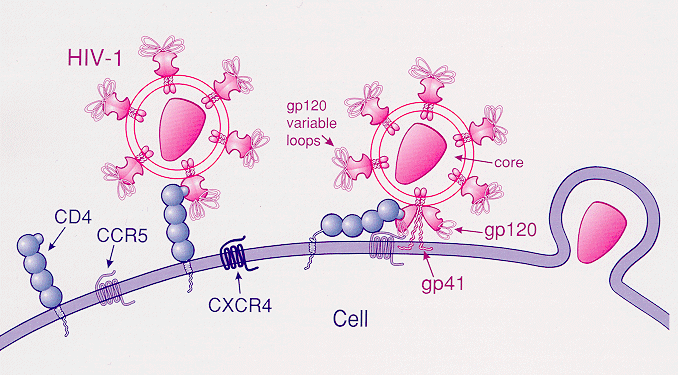
“…apart from the protective effects against HIV infection, the impacts of this mutation, positive or negative, on other diseases are open to debate.”
Xie Y, Zhan S, Ge W, Tang P. The potential risks of C-C chemokine receptor 5-edited babies in bone development.
The reality: The CCR5 mutation is a “truncated protein that cannot be expressed on the cellular surface and therefore is non-functional.” 8 This mutation is derived by a loss of function or allele frequencies potentially associated with earlier diseases, including The Black Death in Europe.6
(I) The mutation is linked to Alzheimer’s disease: “Although the mechanisms of Alzheimer’s disease are diverse and unclear, the past 20 years have witnessed the unprecedented development of the AD inflammation theory. As a key inflammatory receptor family, the C-C chemokine receptor family is a remarkable participant in the cause of Alzheimer’s disease; CCR5 is the most widely studied of this family. CCR5 is essential for viruses to enter cells. The mutations inhibit HIV from entering the cells, which is good for this particular disease but can be detrimental in other cases. This mutation might also impact other inflammatory and immune activities, but this remains largely unknown. New evidence on the inevitably intertwined link between Alzheimer’s disease and CCR5 indicates that CCR5 accelerates the development of Alzheimer’s disease, and few studies have disputed it.3 The role of CCR5 in Alzheimer’s disease remains elusive. However, this intricate relationship will gradually be uncovered as the research progresses. Entanglement of CCR5 and Alzheimer’s Disease. Outside HIV, any additional “good” is unknown and causes memory deficits in mice: “Although the role of CCR5 in immunity and in HIV infection has been studied widely, its role in neuronal plasticity, learning, and memory is not understood. Here, we report that decreasing the function of CCR5 increases MAPK/CREB signaling, long-term potentiation (LTP), and hippocampus-dependent memory in mice, while neuronal CCR5 overexpression causes memory deficits. CCR5 is a suppressor of cortical plasticity and hippocampal learning and memory, so its long-term effects might be detrimental.
(II) Individuals with this mutation have been found to be more adversely impacted by tick-borne illnesses like West Niles.5 “(T)he prevalence of the CCR5 mutation…has been found increased in either West Nile infected subjects or in tick-borne encephalitis.”. 7
(III) The mutation seems driven by allele frequencies not caused by AIDS. It has been found that European people have inherited one copy of the mutated gene. This mutation does not prevent AIDS. “The CCR5-delta 32 is tied primarily to the Eurasia region… and has not been found in Africans, East Asians, or Amerindians..6 Researchers know that “the mutation has been in the population longer than HIV has been infecting people.”. 7 How long the mutation has been in humans depends on which scientist you ask. Estimates range from 700 to 2900 years (ancient assumption). 7 A variation of the level of CCR5 among individuals is believed “to be due to both environmental and genetic aspects.” 7 High levels of CCR5 in countries such as Africa are hypothesized as being due to high levels of parasitic infections.7 The allele frequency (AF) of the deletion (CCR5 mutation) is more than 15% in some European countries like Norway, Estonia, and Latvia, and some Asian and African countries present…lower than 1% Delta32 deletion was discovered in individuals multiply-exposed to HIV that were resistant to the infection and carried two alleles of CCR5-delta32.8 The mutation preexisted AIDS as an allele gene variant! 8
(IV) A CCR5 “knockout” (removal) in mice found impaired induction of memory cells post-influenza infection and increased viral titers in a secondary viral challenge.8 This would lead to a higher susceptibility to viral infections in the future. This indicates that a loss of function of the CCR5 gene is detrimental to the cell’s survival.
Lab verified — derived by broken cellular function
Summary: The CCR5 Delta mutation is caused by the deletion of a single amino acid of the 352 within its protein chain construction. This mutation impacts yet largely (outside HIV research) unknown mechanisms of the function of T-Cells which fight severe disease. This variance works to prohibit HIV (and perhaps other diseases) from entering cells. This mutation has undisputed and inevitable links to Alzheimer’s Disease3 although the exact role the mutation plays remains unknown. Seeing the normal function of this gene (without a mutation) is involved in highly critical functions of the cell regarding immunities its potential negative impacts when mutated remain unknown. This mutation is also linked to learning and memory disabilities due to CCR5 being a suppressor for cortical plasticity.4 Logically, this mutation might also prove to be dysfunctional as it also impacts normal biological functions as well. Outside benefits in stopping inflammatory responses and HIV infection, any “good” are unknown. Supposing the mutation to have occurred 2,500 years ago is clearly deep-time speculation supported (outside known HIV benefits) to be only anecdotal. The “benefit” in protecting against HIV is clear, but it still remains to be subjective (only benefiting those stricken with the disease), and its attributes outside HIV may very well prove to be objectively degradative.
Mutational types referenced:
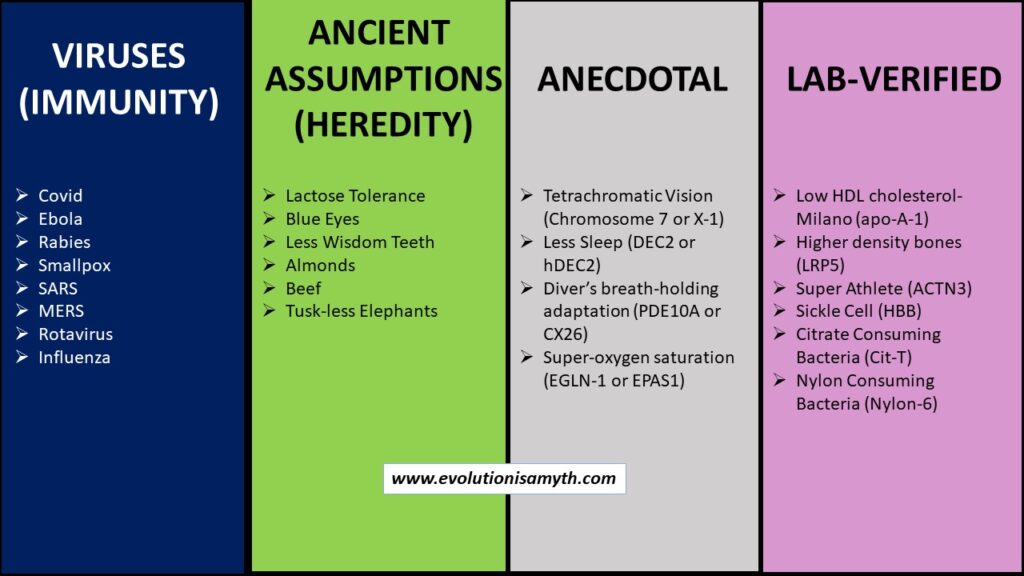
(a) VIRUSES. Virus s are proposed as good evidence for beneficial mutation because they mutate and modify. However, viruses are non-living lipid capsules of degraded genetic material. Viruses are parasites that require a host for metabolism and replication. Most viruses are harmless and are likely genetic debris that remains from a once fully functional genetic process and does not substantiate macro-evolution.
(b) ANCIENT ASSUMPTIONS (Heredity). Unverified mutations, such as lactose tolerance, were speculated to have happened long ago. These are not empirically established but are supposed to have occurred compared to preexisting genetic material. Then, these alterations become the speculation behind presumed mutations of the past. The specific genetic region that affects these attributes is known in the present, but knowledge of the preexisting genetic sequences does nothing to prove a past ancient mutation. Pre-mutational data of a fully sequenced DNA molecule simply does not exist, so that the evidence can infer but not directly substantiate macro-evolution.
(c) ANECDOTAL These have grand claims but often very few examples to confirm, if any. Anecdotal is usually untestable or verifiable by modern lab equipment based on opinions. Many attributes can be spun as beneficial or a fitness gain, but it is anecdotal when data is lacking. A few examples are tuskless African Elephants or tetrachromatic vision. Any anecdotal claims do not and cannot substantiate any inference for macro-evolution.
(d) LAB-VERIFIED. Non-neutral lab-verified mutations render change within the protein folding structure or function with living organisms that display measurable change could confirm the claims of beneficial mutations within macro-evolution. Unfortunately, under very light scrutiny, we found that each of these dozen or so such lab-verified examples was loaded with degradative side effects. Therefore, even lab-verified mutations fail to provide evidence to substantiate macro-evolution. In reality, the preponderance of all genetic data, outside highly dubious and subjective applications, directly opposes such claims revealing that lab-verified mutations, like all others, are degradative.
Note: Genetic trait variances are not mutations at all.
SOURCES:
1- Citation: Ferrero MR, Tavares LP, Garcia CC. The Dual Role of CCR5 in the Course of Influenza Infection: Exploring Treatment Opportunities. Front Immunol. 2022 Jan 20;12:826621. doi: 10.3389/fimmu.2021.826621. PMID: 35126379; PMCID: PMC8810482. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8810482/
2- Xie Y, Zhan S, Ge W, Tang P. The potential risks of C-C chemokine receptor 5-edited babies in bone development. Bone Res. 2019 Jan 29;7:4. doi 10.1038/s41413-019-0044-0. PMID: 30701110; PMCID: PMC6351561.
3- Entanglement of CCR5 and Alzheimer’s Disease; Aging Neurosci., 07 August 2019
Sec. Alzheimer’s Disease and Related Dementias; Li, Tianwen Li & Zhu, Jianhong Aug 2019; https://doi.org/10.3389/fnagi.2019.00209
4- Zhou M, Greenhill S, Huang S, Silva TK, Sano Y, Wu S, Cai Y, Nagaoka Y, Sehgal M, Cai DJ, Lee YS, Fox K, Silva AJ. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory. Elife. 2016 Dec 20;5:e20985. doi: 10.7554/eLife.20985. PMID: 27996938; PMCID: PMC5213777.
5- Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 2010 Jan 15;201(2):178-85. doi 10.1086/649426. PMID: 20025530; PMCID: PMC2934858. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2934858/
6- Paoli, Julia, “HIV Resistant Mutation,” Oct 2013, Scitable by Nature Education; https://www.nature.com/scitable/blog/viruses101/hiv_resistant_mutation/#:~:text=A%20genetic%20mutation%20known%20as,sit%20outside%20of%20the%20cell.
7- Venuti, Assunta; Pastori, Claudia; Lopalco, Lucia, “The Role of Natural Anti-bodies to CC Chemokine Receptor 5 in HIV infection.” Oct 2017; Frontiers in Immunology; Frontiers | The Role of Natural Antibodies to CC Chemokine Receptor 5 in HIV Infection (frontiersin.org)
8- Ferrero MR, Tavares LP, Garcia CC. The Dual Role of CCR5 in the Course of Influenza Infection: Exploring Treatment Opportunities. Front Immunol. 2022 Jan 20;12:826621. doi: 10.3389/fimmu.2021.826621. PMID: 35126379; PMCID: PMC8810482. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8810482/