“Good” Mutation: The “Unbreakable Bones” {LPR5 and LPR6: allelic variants}
The “Good”
When asked for an example of a “good” germline mutation, Naturalists will excitedly point to the “Unbreakable Bones” LPR5 & 6 allelic variant that causes high bone densities in bones. Mutations of these allele variants are thought to provide the carriers with a “superhuman” bone density. This mutation, they claim, provides the fitness gain of stronger bones (based on higher bone densities), which has the mutation dubbed as the “stronger bones” or even the “unbreakable bones” mutation. The lifetime risk of osteoporosis-related fractures is >40% in women and >13% in men. -4
Sounds good! Who wouldn’t want bones of steel? But is it all “good”? No.
The story
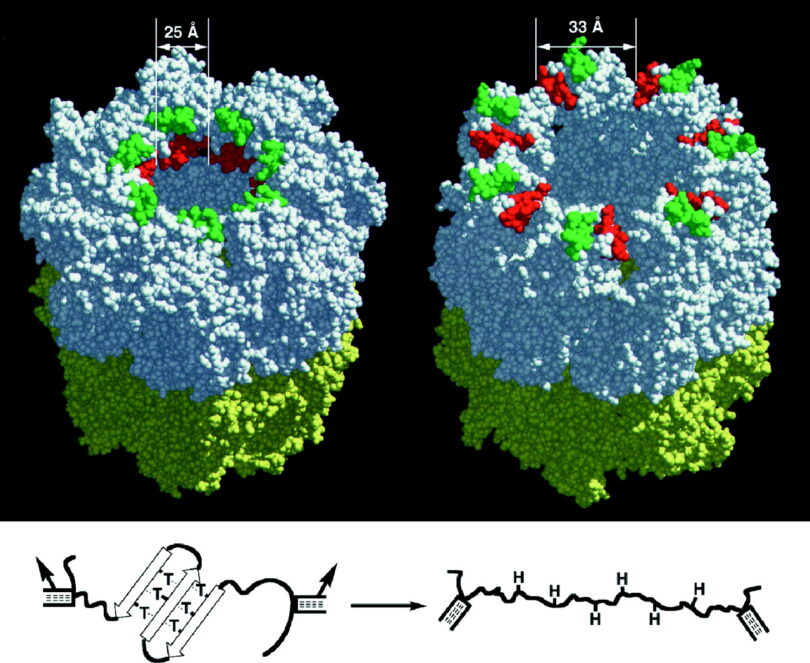
The claim is this beneficial germline mutation, which at present is an allele gene variant (passed to offspring at reproduction), must have emerged long ago due to random mutations (single letter variables) as found within the human genome. The allele variant is thought to have arisen by a fortuitous random mutation (a point mutation or missense mutation) some 500 million years ago, first emerging with the chordate phylum in the Cambrian layers. -1 Wow!
Reproduction
At reproduction, a set of gene variants from the parent’s sex cells, called gametes, emerge with a single allele option for each gamete. Each parent provides one gamete that pairs with the offspring at conception.
These allele variants include thousands of recognizable hereditary traits. Think of people you know and how common they are with their parents or siblings. The most common traits we exaggerate include skin, hair, or eye coloration, which we incorrectly use to divide human beings into races. Genetically, we are only one race: the human race. There are many less obvious but more significant traits passed at reproduction from our parents that include impacting our height, weight, intellect, and more. Bone densities are just another one of these attributes.
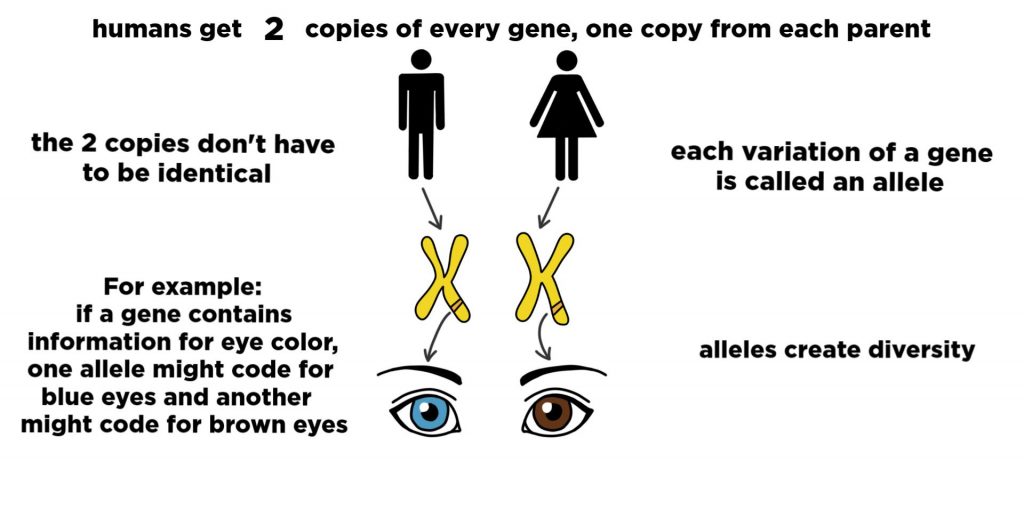
At reproduction, the parents provide offspring with gene variants called alleles. These are selected at conception, which derives variety based on heredity – not mutations. There are several known allele gene options related to bone development in human beings. These variables include many aspects of bone development throughout life that impact many attributes, such as height and other factors, including varying bone densities. These are allele frequencies in the human genome; these are what are believed to have emerged by a mutation long, long ago.
“The result is a thickened mandible and torus palatinus (unfortunately, a bony growth on the roof of your mouth).” -9
“Stature, bone size, and bone mass are interrelated traits with high heritability, but the major genes that govern these phenotypes remain unknown.” -4
Reality
The LRP5 gene (with a sequence allelic variation at the 171st position of the amino acid protein chain or “V171”) is the one that has been thought to impact higher bone density. -9
The specific mechanism underlying various bone disorders, fractures, and diseases like osteoporosis remain poorly understood as applied to allele frequencies. -2 the data varies widely among differing populations, much as lactose tolerance or skin color does. Still, there seems to be a dominant trait that expresses about 65% that emerges as a balanced bone density. -4 This is higher in males and lower in females and varies widely by people groups. -4 Other allelic recessive traits are expressed about 7.4% – 11.7% of the time. -4 Mutations to these variants are rare and often manifest as a disease.
The LPR5-V171 (“mutation”) increases bone density, which is thought to impair the action of typical antagonist “Wnt” pathways, pathways that cells use to express homeostasis and self-renewal. This allelic variant, therefore, seems to increase “Wnt signaling,” which might be a benefit. Findings seem to demonstrate the role of LPR5-V171 alters Wnt pathway function that might cause high bone mass in those with this recessive allelic trait. This discovery has caused the focus of researchers and the claim to fame of the allele variant thought to have emerged by an ancient mutation to seek out this variant as a potential target for research and prevention for osteoporosis and other bone disorders. -9
Notably, reduction of “Wnt signaling” (reduction leads to a host of problems from loss of function to cancer-7) directly causes many disorders, including childhood-onset osteoporosis called “Idiopathic juvenile osteoporosis” (IJO)-6. The first symptoms of IJO appear well before puberty, and the main symptoms include reduced bone mineral density (BMD), vertebral compression fractures, and metaphyseal fractures in the long bones. The fractures lead to bone pain and impaired mobility [-5, -6]. This mutation exhibits “dangerous and unwanted side effects.” -6 Increasing “Wnt signaling” seems to provide the gene some bone density benefits.
The low-density lipoprotein receptor-related protein LPR5 gene has been linked to allelic variations in bone mineral density and types of bone diseases. -2
“The LRP5 gene contributes to subsequent reduction of bone mineral density (BMD), indicating a dominant negative effect on bone mass, which would lead to various bone diseases.” -2
Recent research has shown that the LRP5 gene reduces bone mineral density (BMD) as a loss-of-function (if it has indeed emerged long ago as a “missense” or “point” mutation). Doesn’t the opposite seem much more likely? Consider that the dominant trait found in “normal” bone density (with a 65% allele frequency in the human population) compared to the rarer recessive strains which derive reduced BMD. Which is most likely the loss of function? It is obvious, but it does not tell the narrative of Darwinism as nicely as the opposite.
Genetic diseases
We know that countless genetic mutations lead to an array of diseases. Variants of the LRP5 gene are also known to lead to various bone disorders, from osteoporosis to high bone mass (HBM) disorders or “osteogenesis imperfecta,” which are rare but manifest as bone dysplasia, bone fragility, and increased bone densities, which serves as a “loss-of-function” mutations caused by osteoporosis syndrome (OPPG). This syndrome is characterized by early-onset bone fragility and complications in eye development up to blindness. -4, -5
In experiments with mice, the LRP5 mutation expressed a low bone mass phenotype that exhibited decreased osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization. -5
Associations have also been reported between the LRP5 gene and polymorphisms (meaning effects that negatively affect bone mass and size) in those affected by the genetic mutation. -5 These mutations can directly cause a loss of function (in the) low-density lipoprotein receptor…(LRP5) of its inhibitor Sclerostin (SOST).
Rare inherited autosomal dominant mutations (a degradative genetic mutation carried by each parent) significantly increase the likelihood of degradative side effects. -6 Findings indicate unopposed “Wnt signaling” results due to ‘loss of action of a normal antagonist,’ which is the molecular mechanism that accounts for detrimental effects due to the mutation.
Loss of function mutations in the LRP5 has been implicated in osteoporosis-pseudoglioma (a rare syndrome characterized by severe thinning of the bones (osteoporosis) and eye abnormalities that lead to vision loss) and other “high-bone-mass” syndromes. They serve effectively as genetic disease profiles. Studies have elucidated that the allele distribution of the LRP5 gene variants (outside congenital disease) does seem to contribute to bone mass determination in the general population. However, it should be noted that, unfortunately, the associations vary widely based on the subject’s ethnicity, biological sex, age, body mass, collagen production, vitamin D receptors, various hormone levels, and perhaps even height. -3, -4
Genes passed with known mutations to the allele variants – not new ones which directly cause thousands of diseases, including cancers, tumors, congenital disorders, illnesses like Autism, deformities, and even premature death.
The claim is plausible– just not substantiated by evidence.
The (supposed) beneficial germline mutation is hypothesized to have emerged 500 million years ago during the Cambrian. While this claim is plausible, it remains unsubstantiated by physical genetic evidence. Neither the evidence known in the present gene sequences regarding bones nor the fragmented genetic evidence rarely found in fossils defend the claim of a beneficial mutation. -8 The genetic evidence in the present is well known, but its persistence tells us nothing about its origin. And the fossils are just too fragile and incomplete to substantiate an ancient genetic sequence and “permutational” condition. In other words, the claim is just an inference made without physical evidence.
Conclusions
Just as eye coloration in humans is based on allelic traits that are supposed to have emerged many thousands of years ago, so are those of the bone density LPR5 and LPR6. These are considered over 500 million years old, a vastly weak hypothesis because, as we have discussed, it is not based on evidence but essentially circular reasoning. The assumption that superior bone densities as an allele gene variant (aka “unbreakable bones”) first emerged as a beneficial germline mutation is largely unsupported by direct evidence. As far as we know, humans have always had varying allele variants -9 for many physiological varieties, including bone densities.
Bone Density varies based on which allele variant the offspring receives. Whether an ancient mutation first formed these allelic attributes is an inference. We know that allele frequencies are expressed as either dominant or recessive traits. The human genome has genetic variability throughout its volume. The gene that impacts bone density is no different.
What does this mean?
Allele frequencies confer benefits to offspring as we observe them (provided they are free of genetic disease-causing mutations- congenital mutations). Benefits such as eye, hair, and skin tones. Also, height, intellect, and countless other attributes. Naturalists presume that each of these beneficial traits emerged by various random mutations over eons of time. That is the presumption.
The mutation behind the supposed “unbreakable bones” happened 500 million years ago. Okay. You must admit that these are fantastical hypotheses.
We empirically observe that known genetic mutations in the present cause disease and premature death, which logically implies that our genetics today are better than yesterday, and tomorrow, they will be worse. This is what evidence shows that humans are accumulating about 100 new genetic mutations per generation. -10 This has been coined as “genetic entropy” by geneticist Dr. John C. Sanford of Cornell University. -10
The claim that mutations observed in the present wreaking havoc on life somehow conferred benefits to form novel allele frequencies and body forms is farfetched.
Intelligent Design describes what we observe in heredity. Heredity explains everything we keep in the biology period. Happenstance, random mutations, and atomic predestination over time form the hypothesis of Naturalism. Intelligent Design would find that all the goodness that continues in life, due to a persistence of high fidelity in DNA replication essentially free of mutations, explains life best.
The Claim of “Evolution” (overview)
Beneficial germline mutations require evolution (aka universal common ancestry, Modern Synthesis, Neo-Darwinism, Darwinian evolution, etc.) to have a mechanism to deliver the metamorphosis of the novel (all new) organisms (phyla, not species). The claim is that such beneficial germline mutations impacted the gametes of life forms. This explains how single-cellular life forms like bacteria eventually birthed multi-cellular life forms like fishes, frogs, reptiles to mammals, and the pinnacle monkeys to humans.
Evolution works by Natural Selection
Upon light inspection, Natural Selection is comprised of two distinct phenomena: (1) Reproduction and (2) “Beneficial” germline mutations that derive physiological gains to viable offspring, ultimately as a means of deriving all the vast variety of life as we observe it today. Below, the diagram explains this as The Modern Synthesis.
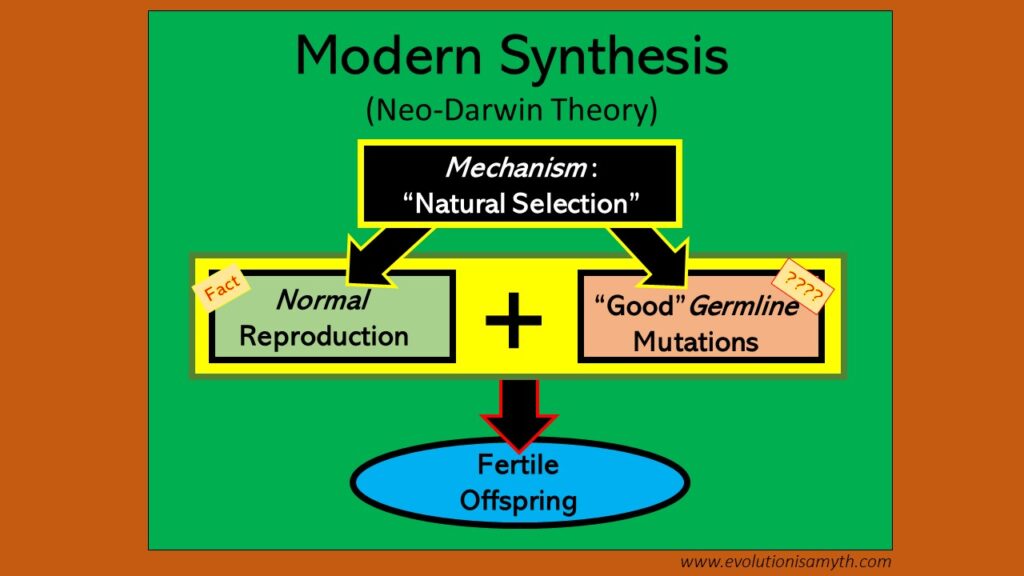
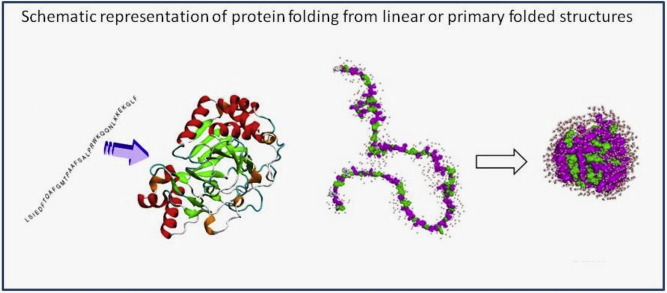
How biology makes living things
Biology works within a three-dimensional world of interlocking and prohibitive shapes. Proteins work in teams throughout biology. The assembly of the protein chains is derived from highly specified genetic codes copied by RNA from DNA. This code translates into protein chains. The shapes of proteins are formed by a massively complex folding process.
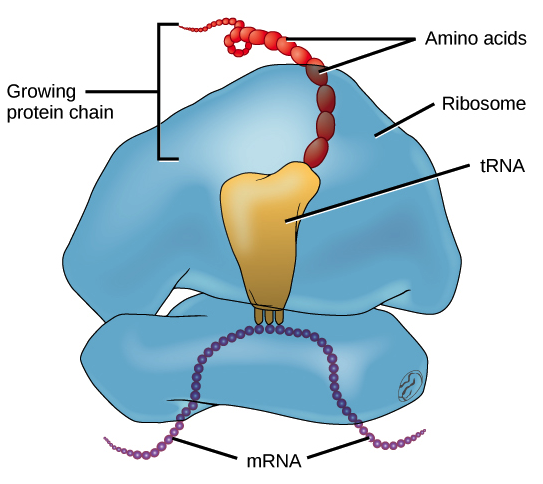
A copy of the gene (mRNA) is delivered to the ribosome, translating the information into a chain of amino acids like a ticker tape. Each of the twenty amino acids is delivered to the site by specialized transporters (called tRNA). The protein chain (the “gene”) uses three-letter segments called “codons.” The arrangement of these codons determines exactly how the protein will fold (what shape the protein ultimately becomes).
Note: The average length of a protein chain is about 350 with a maximum for a flexible muscle fiber called Titin which is well over 35,000.
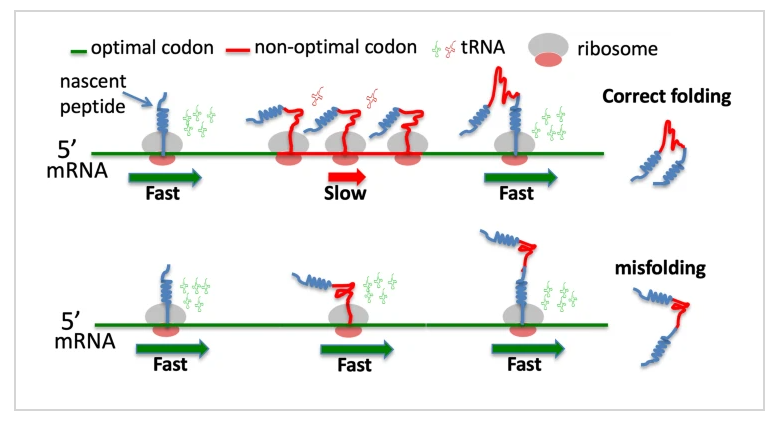
How mutations impact biology
Some codon sequences do not affect the protein’s folding and can pass as neutral, although recent research is finding that maybe there is no such thing as silent mutations– even these are harmful. -6 Those that affect the folding of the protein derive degradation by deleterious effects, causing malformed and unusable proteins in the living cell. This is why even those mutations that can subjectively be thought to provide a benefit ultimately derive dysfunction, disease, and death. This phenomenon is called an unsolvable biological reality called Levinthals Paradox.
The problem of protein folding is one of the most important problems of molecular biology. A central problem (the so called Levinthal’s paradox) is that the protein is first synthesized as a linear molecule that must reach its native conformation in a short time (on the order of seconds or less). The protein can only perform its functions in this (often single) conformation. The problem, however, is that the number of possible conformational states is exponentially large for a long protein molecule. Despite almost 30 years of attempts to resolve this paradox, a solution has not yet been found.
https://www.sciencedirect.com/science/article/abs/pii/S0079610717300846#preview-section-references. Bold is mine.
Mutations (that are not neutral) alter the three-dimensional shape of proteins.
Proteins work in teams, so the newly hatched mutant has no enzyme or transporter partner. It is unusable to the cell. This is how cancers and tumors form. Biology does not function in a bubble; foreign entities (mutated proteins) can derive a cascade of interrelated and unintended side effects. Malformed proteins are devastating to biological function. Mutations directly cause countless diseases, cancers, deformities, tumors, congenital disorders, disabilities like Autism, and premature fetal death.
This is why seeking out any supposed “beneficial” mutation, regardless of how subjective that benefit might be, is like looking for a needle inside a haystack, a haystack bigger than the entire Milky Way.
1- MacDonald BT, Semenov MV, Huang H, He X. Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PLoS One. 2011;6(8):e23537. doi: 10.1371/journal.pone.0023537. Epub 2011 Aug 24. PMID: 21887268; PMCID: PMC3160902. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3160902/
2- Xu GY, Qiu Y, Mao HJ. Common polymorphism in the LRP5 gene may increase the risk of bone fracture and osteoporosis. Biomed Res Int. 2014;2014:290531. doi: 10.1155/2014/290531. Epub 2014 Dec 14. PMID: 25580429; PMCID: PMC4279179. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279179/
3- Mizuguchi, T., Furuta, I., Watanabe, Y. et al. LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J Hum Genet 49, 80–86 (2004). https://doi.org/10.1007/s10038-003-0111-6; https://www.nature.com/articles/jhg200413#citeas
4- Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004 May;74(5):866-75. doi: 10.1086/420771. Epub 2004 Apr 7. PMID: 15077203; PMCID: PMC1181981. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1181981/
5- Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity – BMC Medical Genetics
6- Korvala, J., Jüppner, H., Mäkitie, O. et al. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet 13, 26 (2012). https://doi.org/10.1186/1471-2350-13-26; https://www.science.org/doi/10.1126/scitranslmed.aau7137
7- Liu, J., Xiao, Q., Xiao, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sig Transduct Target Ther 7, 3 (2022). https://doi.org/10.1038/s41392-021-00762-6; https://www.nature.com/articles/s41392-021-00762-6#citeas
8- Mélanie Pruvost, Reinhard Schwarz, Virginia Bessa Correia, +5 “Freshly excavated fossil bones are best for amplification of ancient DNA” Jan, 2007, 104 (3) 739-744; https://doi.org/10.1073/pnas.0610257104
9- High Bone Density Due to a Mutation in LDL-Receptor–Related Protein 5, Lynn M. Boyden, Ph.D., Junhao Mao, Ph.D., Joseph Belsky, M.D., lyle Mitzner, M.D., Anita Farhi, R.N., Mary A. Mitnick, Ph.D., Dianqing Wu, Ph.D., Karl Insogna, M.D., and Richard P. Lifton, M.D., Ph.D.May 16, 2002 N Engl J Med 2002; 346:1513-1521, DOI: 10.1056/NEJMoa013444; https://www.nejm.org/doi/full/10.1056/nejmoa013444
10- J. C. Sanford, Book “Genetic Entropy and the Mystery of the Genome”, 3rd ed. (Waterloo, NY: FMS Publications, 2008).